Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1725
Research Article(ISSN: 2641-1725) 
An Anti-Inflammatory Adrenocorticalsteroid Hydrocortisonesodiumsuccinate, Hydrocortisone Preparation and Validated for the Confirmation/ Determination and Quantification of Hydrocortisone Volume 1 - Issue 5
Krishnasarma Pathy*
- Head R&D-QC/QA, IPL Research Centre Lucknow, India
Received: September 12, 2018; Published: September 21, 2018
*Corresponding author: Krishnasarma pathy, Head R&D-QC/ QA, IPL Research Centre Lucknow, India
DOI: 10.32474/LOJMS.2018.01.000121
Abstract
Hydrocortisone is a naturally occurring corticosteroid hormone secreted by the adrenal cortex and released during times of stress. The synthetic drug is employed in the management of inflammatory and rheumatoid diseases, allergic conditions, and autoimmune disorders such as Addison’s disease (adrenal insufficiency disease). Hydrocortisone is commercially available in pharmaceutical formulations such as tablets, capsules, creams, ointments, and injections. Hydrocortisone may exist commercially as the unchanged hormone or as the acetate, cypionate, sodium phosphate, butyrate, valerate, and the sodium succinate forms. Preparation of the present paper provides a method for preparation of hydrocortisone , method for preparation of hydrogenation cortisone sodium succinate reaction temperature is low, fast, easy to produce during the reaction of other impurities, fewer side effects and was concentrated, high yield, where water and other solvents content is low, to lay the foundation for post-drying, hydrogenation of the hydrocortisone sodium succinate prepared in line with CP2005 and USP28 standards, for clinical use and also an isocratic sensitive and precise reverse phase high-performance liquid chromatography (RP-HPLC) method was developed and validated for the determination and quantification of hydrocortisone in controlled-release and conventional (tablets and injections) pharmaceutical preparations. Chromatographic separation was achieved on an ODS (C18), 5m, 4.6 × 150mm, with an isocratic elution using a freshly prepared mobile phase of composition methanol: water: acetic acid (60: 30: 10, v/v/v) at a flow rate of 1.0ml/min.
The detection of the drug was successfully achieved at a wavelength of 254 nm. The retention time obtained for the drug was 2.26 min. The proposed method produced linear detectable responses in the concentration range of 0.02 to 0.4 mg/ml of hydrocortisone. High recoveries of 98-101% were attained at concentration levels of 80%, 100%, and 120%. The intraday and interlay precision (RSD) were 0.19-0.55% and 0.33-0.71%, respectively. A comparison of hydrocortisone analyses data from the developed method and the official USP method showed no significant difference () at a 95% confidence interval. The method was successfully applied to the determination and quantification of hydrocortisone in six controlled-release and fifteen conventional release pharmaceutical preparations.
Introduction
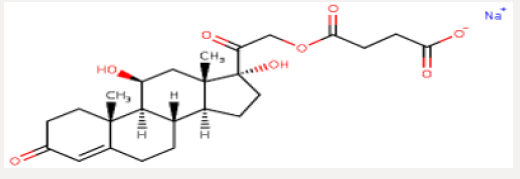
Hydrocortisone sodium succinate is an anti-inflammatory adrenocortical steroid. This highly water-soluble sodium succinate ester of hydrocortisone permits the immediate intravenous administration of high doses of hydrocortisone in a small volume of diluent and is particularly useful where high blood levels of hydrocortisone are required rapidly. A-Hydrocort sterile powder is available for intravenous or intramuscular administration. 100 mg− Vials containing hydrocortisone sodium succinate equivalent to 100 mg hydrocortisone, also 0.8 mg monobasic sodium phosphate anhydrous, 8.73 mg dibasic sodium phosphate anhydrous. When necessary, the pH was adjusted with sodium hydroxide so that the pH of the reconstituted solution is within the USP specified range of 7 to 8 (Figure 1).
Preparation of Solutions
100mg - For intravenous or intramuscular injection, prepare solution by aseptically adding not more than 2mL of Bacteriostatic Water for Injection or Bacteriostatic Sodium Chloride Injection to the contents of one vial. Further dilution is not necessary for intravenous or intramuscular injection. For intravenous infusion, first prepare solution by adding not more than 2mL of Bacteriostatic Water for Injection to the vial; this solution may then be added to 100 to 1000mL of the following: 5% dextrose in water (or isotonic saline solution or 5% dextrose in isotonic saline solution if patient is not on sodium restriction). In cases where administration of a small volume of fluid is desirable, 100 mg of hydrocortisone sodium succinate may be added to 50mL of the above diluents. The resulting solutions are stable for at least 4 hours and may be administered either directly or by IV piggyback. When reconstituted as directed, pH’s of the solutions range from 7 to 8 and the tonicities are: 100mg vial, .36 osmolar (Isotonic saline = .28 osmolar.) For intravenous or intramuscular injection, vial should be reconstituted with Bacteriostatic Water for Injection or Bacteriostatic Sodium Chloride.
For intravenous infusion, vial should be reconstituted with Bacteriostatic Water for Injection. Hydrocortisone and hydrocortisone salts are known for use as anti-inflammatory agents. It is common to use these active substances in the treatment of asthma in particular, endocrine disorders, dermatological disorders and allergic conditions, and acute adrenal insufficiency. Hydrocortisone and its salts belong to the family of corticosteroids. Hydrocortisone and its salts are susceptible to oxidation. Therefore, pharmaceutical solutions of these active ingredients always include at least one antioxidant. However, antioxidants used in these pharmaceutical solutions are themselves unstable. Therefore, it is known that these pharmaceutical solutions comprise a 2nd antioxidant is disodium ethylenediamine tetraacetate (hereinafter abbreviated as “disodium EDTA”). The disodium EDTA serves to stabilize (or otherwise preserve) the antioxidant which is itself chosen to keep the hydrocortisone thus preserving oxidation.
Preparation of Hydrocortisone
Hydrocortisone Step A : 3 - [(1,2-ethanediyl) mercaptole] cyclic Androst-4-ene-3,11,17-trione.
a) Androst-4-ene-3,11,17-trione: Was mixed under inert gas at room temperature 1.05g of 9alpha-bromo 11beta-hydroxy androst-4-ene-3,17-dione, 7.5cm3 of ethyl acetate and 2.5cc of ethylene glycol. Refluxed under stirring for 10 hours, cooled and then added 10cm3 of 2N hydrochloric acid and 10cm3 of water acid, stirred for 20 hours then remove the ethyl acetate under reduced pressure. After salting out with sodium chloride is cooled to 0°C, the crystals were separated, washed with water and dried. Were repeated with methylene chloride, add ethyl acetate, methylene chloride is evaporated, ice the solution and the crystals were separated and dried. 0.52g of the expected product, mp=221°C. By extraction of the aqueous phase with methylene chloride and chromatography on silica of the crude product, eluting with mixture of ethyl chloride méthylèneacétate (95-5) is obtained, after crystallization from ethyl acetate, 0.097g of expected product melting at 220°C.
b) 3 - [(1,2-ethanediyl) mercaptole] cyclic androst-4- ene-3,11,17-trione: Is room temperature under an inert gas mixture 100cm3 of methanol, 5g of product obtained as described above, 1.8cm3 of ethanedithiol and 2.5cm3 boron trifluoride etherate. After 1h 30 with stirring the methanol is evaporated, taken up in methylene chloride, washed with saturated aqueous sodium bicarbonate, water and dried and evaporated to dryness. The product was crystallized obtained in hexane and obtained 5.99g of expected product melting at 160°C.
NMR Spectrum (CDCl3 90 MHZ ppm)
18-CH3: 0.85; 19--CH3: 1.27; thioketal: 3.17 to 3.47; H4: 5.6 IR Spectrum (CHCl3)
Absence of Δ4 3-one; absorptions at 1645cm-1 (C=C), 1709cm- 1 (C=0 in 11-position), 1740cm-1 (C=0 in 17-position).
A. Step A: 3-[(1,2-ethanediyl) mercaptole] cyclic Androst- 4-ene-3,11,17-trione under inert gas mixture 4g of 9alpha-bromo 11beta-hydroxy androst-4-ene-3,20-dione and 40cm3 of ethyl acetate and then added at ambient temperature 0.9cm3 of ethane dithiol. then slowly added 0.09cm3 of hydrochloric acid at 22° Bé then stirred for 6 hours. Is then introduced 9.3cc of ethylene glycol was refluxed for 20 hours and then cooled to 20°C and pour the reaction mixture into a mixture of 40cm3 of 2N hydrochloric acid and 40cm3 of water. stirred for 16 hours then removed ethyl acetate under reduced pressure (20mm/Hg) at 35°C maximum then the suspension is cooled to 0,+5°C, stirred for 1 hour and the crystals were separated. Washed with water and then dried and chromatographed on silica eluting with hexane-dioxane mixture (9-1). Is obtained 3.2g of the expected product.
NMR Spectrum (CDCl3 300 MHz ppm): 18--CH3: 0.84 (s); 19-- CH3: 1.26 (s); thioketal: 3.15 to 3.4; H4: 5.57; skeleton: 1.1 to 2.61(m)
IR Spectrum (CHCl3): Absorptions at 1741-1709 cm-1 (ketones); 1641 cm-1 (C=C)
B. Step B: Methyl 20-chloro-3,3,1,2-ethanediyl-bis(thio) !-Δ4,17(20)=pregnadien-11-one-21-oate 20-chloro-3,3-[1,2- ethanediyl bis (thio)]-11-oxo-pregna-4,17(20)-dien methyl-21- oate under an inert atmosphere is mixed 100cm3 of tetrahydrofuran and 12.55g of zinc powder. was slowly added at -10°/-15°C, 7.9cm3 of titanium tetrachloride and then a mixture of 100cm3 of tetrahydrofuran, 8.6cm3 of methyl trichloroacetate and 18g of product obtained as described in Step A or A ‘. The temperature was allowed to rise and then kept stirring at room temperature for 1h 30min then added at + 10/+ 5°C, 100cm3 of water-pyridine (4- 1), stirred for 1 hour while allowing the temperature to rise then added 100cm3 of concentrated hydrochloric acid-water mixture (6-4). Stirred for 15 minutes, extracted with methylene chloride, the organic phase washed with water, dried and the solvent evaporated. The crystals were dissolved in methylene chloride, added isopropyl ether, evaporating the methylene chloride, cooled and the crystals were separated. The mother liquors were chromatographed on silica eluting with cyclohexane-ethyl acetate mixture (8-2). Is recovered in total 21.8 g of expected product F≈175°C.
a) IR Spectrum (CHCl3)
Absorptions at 1715cm-1 and 1730cm-1 (C=0) max. 1705 cm-1 1643cm-1 (C=C Δ4) and 1610cm-1 (C=C) NMR Spectrum (CDCl3 90 MHz ppm) Mixture of isomers 20 Cl 18--CH3: 1.02-0.98; 19--CH3: 1.25; thioketal: 3.33; CH3 --ester: 3.83-3.82; H4: 5.58.
C. Step C: 20-phenoxy-3,3- [1,2-ethanediyl bis (thio)] 11-oxopregna- 4,17 (20) -dien methyl-21-oate. Mixture is brought to reflux under an inert atmosphere a mixture of 18 g of phenol, 150 cm3 of butanone, 30 g of product obtained according to the procedure described in Step B, and 17.7 g of potassium carbonate. After 16 hours the mixture was poured into a mixture of 100 cm3 of water, 90 g ice and 10 cm3 of 10N sodium hydroxide. Extracted with methylene chloride, the organic phase washed with water and concentrated. Is taken up with methanol, allowed to cool slowly and the crystals were separated and dried. into two streams are obtained 27.4 g of the expected product F≈208-210 °C.
a) IR Spectrum (CHCl3): Absorption at 1592-1491 cm-1 (Aromatic C6 H5 -0-type); 1714-1705 cm-1 (C=0); 1646 cm-1 (C=C) NMR Spectrum (CDCl3 90 MHz ppm)
18-CH3: 0.9; 19-CH3: 1.21; thioketal: 3.33; CH3 ester: 3.63; H4: 5.58; C6 H5: 6.81 to 7.39 Mixture of isomers 20-0-C6 H5
D. Step D: (11beta) 3,3 - [(1,2-ethanediyl) mercaptole] cyclic 20-phenoxy-11,21-dihydroxy-pregna-4,17 (20) -dien-3-one. Is under an inert gas mixture 20 g of product obtained in Stage C and 200 cm3 of toluene, cooled to -25 °C and slowly added 110 cm3 of a 20% solution of diisobutyl aluminum hydride in toluene. The temperature was allowed to rise to + 10 °C, stirred for 1 hour then cooled again to -15 °C was slowly added 10 cm3 of methanol, allowed to rise to 0 °C and slowly added 200 cc of 2N hydrochloric acid. After decanting, the organic phase washed with water, dried and the solvent evaporated. The residue was chromatographed on silica eluting with toluene-ethyl acetate mixture (9-1). One obtains 17.4 g of expected product.
a) IR Spectrum (CHCl3):
Absorption at 1490-1596 cm-1 (C6 H5 -0-C); 1644 cm-1 (C=C) Δ4) and 1682 cm-1(C=C); 3612 cm-1 (free OH) NMR Spectrum (CDCl3 -C5 D5 N) (90 MHz ppm)
E. Step E: (11beta) 11,21-dihydroxy 20-phenoxypregna- 4,17 (20) dien-3-one.Is dissolved 5 g of the product obtained above in 10 cm3 of methylene chloride and 30 cm3 of methanol, 2.5 cm3 of deionized water and 0.3 g of iodine. There is obtained a pH of 1.5 and is introduced in 15 minutes 1.4 cc of hydrogen peroxide at 50%. Neutralized oxidizing power by addition of 2 g of sodium thiosulphate. Then 5 g of clarcel, filtered and concentrated to dryness under reduced pressure. The dry extract is dissolved in methylene chloride, washed with a solution of 1g sodium thiosulfate in 25 cm3 of water, decanted, dried and concentrated to dryness under reduced pressure to obtain 4.8 g of the expected crude product .1.8 g of purified product by chromatography on silica (eluent methylene chloride-isopropanol (97.5-2.5)) to obtain 1.7 g of desired product. F = 188 °C.
a) IR Spectrum (CHCl3):
Absorptions at 3613 cm-1 (OH); 1662, 1617 and 868 cm-1 (Δ4 -3- oxo); 1597-1491 cm-1 (--0--C6 H5)
NMR Spectrum (CDCl3 -C5 D5 N--90 MHz ppm)
18-CH3 : 1.17; 19--CH3 Z: 1.42; H11 : 4.27; H4 : 5.67; CH2 OH: 4.11; C6 H5 : 6.87 to 7.37
F. Step F: (11beta) 11,21-dihydroxy 17,20-epoxy 20-phenoxy-pregna-4-en-3-one.under an inert gas are mixed 1.0 g of product obtained in Stage E, 10 cm3 of ethyl acetate and 5 cm3 of water are added. there is added 0.5 g of disodium phosphate, 0.65 g of perphthalic acid and 0.75 cc of 50% hydrogen peroxide, stirred for 3 hours 15 minutes, add 0.15 g of disodium phosphate and 0, 20 g of perphthalic acid and stirred for 1 hour 15 minutes. then added 20 cm3 of ethyl acetate and 9 cc of 1N sodium hydroxide, stirred 5 minutes, decanting, washing the organic phase with water and water containing sodium bisulfite and sulfuric acid 1N, dried and evaporated to dryness. Was obtained 1.05 g of expected crude and unstable product was used as is for the following stage. Rf: 0.28 (silica--CH2 Cl2 /Dioxane--90/10).
IR Spectrum (CHCl3)
Absorption at 3613 cm-1 (OH); 1662 and 1616 cm-1 (Δ4 -3-one); 1600, 1590 and 1494 cm-1 (aromatic)
NMR Spectrum (CDCl3 300 MHz ppm)
18--CH3 : 1.29 (s); 19--CH3 : 1.43 (s); --C--CH2 --0--: 3.49 (dd) and 4.20 (dd); H11 eq.: 4.32; H4 : 5.68; H of O-C6 H5 : para 7.06 (t), ortho 7.13 (d) and meta 7.29 (t).
G. STEP G: Hydrocortisone 5 ml of methanol, 3 ml of water and 0.08 ml of 1N sulfuric acid (pH 2) were mixed together under an inert gas atmosphere and after 0.525 g of the product of Step F were added at ambient temperature, the mixture was stirred for 16 hours. The mixture was neutralized by the addition of sodium bicarbonate and extraction was carried out with methylene chloride. The organic phase was dried and evaporated to dryness. The residue was taken up in hot methylene chloride with 5% methanol, then concentrated until the start of crystallization. After cooling, the crystals were separated and dried. The mother liquors were concentrated, and the residue was chromatographed on silica eluting with a methylene chloride--methanol (95-5) mixture to obtain in total 0.318 g of the expected hydrocortisone melting at 224°C. and having a specific rotation of α!20D =+164°±2° 5 (C=1% in ethanol).
a) IR Spectrum (nujol):
Absorption at 3430 cm-1 (OH), 1710 cm-1 (C=0), 1642, 1630 and 1610 cm-1 (Δ4 -3-one).
Hydrocortisone Hemisuccinate
Preparation: A mixture of1Kg hydrocortisone, 1.9kg dimethyl formamide into a reaction tank dried ‘ and stirred at 20-25’C stirred solution clear. Slowly cooling to 100C, put 0.22Kg triethylamine was slowly added 0.8Kg succinic anhydride, control temperature of 15 C reaction maintain 3,5 hours. In the mixing state has been lowered to 2 diluted. C›s 15Kg purified water, 5-80C stand for 3 hours, filtered the material, color is white or off-white powder. The yield was 92.64%, a melting point of 170 °C
Example 2: Hydrocortisone sodium succinate Synthesis: (TC, completely dissolved with stirring for about 50min the method of Example 1 to give hydrocortisone succinate 50g, the ratio of the amount of 200ml of purified water was added, dissolution temperature Ol plus carbonate. sodium 9g, slowly add the ratio of the amount of acetone 100ml, about 2ml / minute rate dropped, after the addition was complete, the temperature control at Ol (TC, thermal reaction 2h, gradually dissolving clear, plus carbon 2.5g, bleaching 30min, filter bucket filtered, concentrated under reduced pressure, T “3 (TC, vacuum should” -0.08MPa, hydrocortisone sodium succinate and concentrated to 46.32g, color is white or offwhite powder. The yield was 92.64%, a melting point of 170 °C.
Example 3: Hydrocortisone sodium succinate Synthesis: Example 1 obtained hydrocortisone succinate 50g, the ratio of the amount of added 165ml of purified water dissolution temperature of 10-20 °C, stirred for about 40min completely dissolved. Sodium hydroxide 4.3g, slowly increase the ratio of the amount of acetone 100ml, about 2ml / minute rate dropped, after the addition was complete, the temperature control at 10-20 °C, the reaction was kept 1.5h, gradually dissolving clear added carbon 2.5g , bleaching 30min, filter bucket filtered, concentrated under reduced pressure, T “3 (TC, vacuum should” -0.08MPa, hydrocortisone sodium succinate and concentrated to 45.86g, color is white or off-white powder. The yield 91.72%, m.p. 170 °C.
Example 4
Hydrocortisone sodium succinate Synthesis:
a) Example 1 obtained hydrocortisone succinate 50g, the ratio of the amount of 165ml of purified water was added, the reaction temperature is 10-20 °C, stirred for about 40min completely dissolved.Plus sodium bicarbonate 9g, slowly add acetone 100ml, about 2ml / minute dropped, after the addition was complete, the temperature control at 10-20 °C, the reaction was kept 1.5h, gradually dissolving clear, plus carbon 2.5g, bleaching 30min, filter bucket filtered, concentrated under reduced pressure, T “30’C, vacuum should” -0.08MPa, hydrocortisone sodium succinate and concentrated to 47.25g, color is white or off-white powder. The yield was 94.5%, mp 170. 5 °C
Hydrocortisone sodium succinate Synthesis: Example 1 obtained hydrocortisone succinate 50g, the ratio of the amount of 165ml of purified water was added, the reaction temperature was 20-3 (TC, stirred for about 30min completely dissolved. plus sodium bicarbonate 9g, slowly add the ratio of the amount of acetone 100ml, temperature and reaction lh, gradually dissolving clear added carbon 2.5g, bleaching 30min, filter bucket filtered, concentrated under reduced pressure, T “3 (TC, vacuum should” -0.08 MPa, hydrocortisone sodium succinate and concentrated to 47.4g, the color is white or off-white powder. The yield was 94.8%, a melting point of 171 °C.
Hydrocortisone sodium succinate synthesis: by the method of Example 1 to give hydrocortisone Pine succinate 50g, 165ml of purified water was added, the reaction temperature is 30-40 °C, stirred for about 20min completely dissolved. Plus sodium bicarbonate 9g, slowly add the ratio of the amount of acetone 100ml, about 2ml / minute rate dropped, after the addition was complete, the temperature control at 10-15 °C, the reaction was kept lh, gradually dissolving clear added carbon 2.5g, bleaching 30min, filter bucket filtered, concentrated under reduced pressure, T “30. C, the vacuum should «-0.08MPa, hydrocortisone sodium succinate and concentrated to 44.71g, color is white or off-white powder. The yield was 89.42%, the melting point of 17 (TC).
b) Example 3: Hydrocortisone sodium succinate synthesis: by the method of Example 1 to give hydrocortisone Pine succinate 50g, 165ml of purified water was added, the reaction temperature was 40-5 (TC, dissolved 20min. Add sodium bicarbonate 9g, slowly add acetone 100ml, approximately 2ml / min speed of the dropwise addition, ‘After the addition was complete, Temperature control in 10-15’C, heat the reaction 1.5h, gradually dissolving clear, plus carbon 2.5g, bleaching 30min, filter bucket filtered, concentrated under reduced pressure, T “3 (TC, vacuum should” -0.08MPa, concentrated to hydrocortisone sodium succinate 46.32§, the color is white or off-white powder. The yield was 92.64%, the melting point of 170. 5 .C. yield 93%, mp 171 °C.
After a detailed description of the preferred embodiments, those skilled in the art can clearly understand, ‘without departing from the scope and spirit of the above-mentioned patent applications under various changes and modifications, all based on technical essence of the invention of the above embodiments Any changes made simple equivalent change and modifications are within the scope of the present invention, the technical solution. And the present invention is also not limited to the examples cited in the specification embodiments.
A search of the literature revealed that few analytical methods such as ultraviolet spectrophotometry [2], electrokinetic capillary chromatography [3,4], photochemically enhanced fluorescence [5], thin-layer chromatography- (TLC-) densitometry [1,6], and high-performance thin-layer chromatography (HPTLC) [7] have been used for the estimation of hydrocortisone in pharmaceutical formulations. However, modern chromatographic techniques such as high-performance liquid chromatography (HPLC) [8-12] and ultraperformance liquid chromatography (UPLC) [13] are preferentially employed for the determination of hydrocortisone in conventional pharmaceutical products such as tablets, creams, and injections. The different HPLC methods employ different chromatographic conditions including the use of complex and sometimes expensive solvent systems. Thus, access to some of these solvent systems in resource-poor countries in the developing countries can be challenging. The development of a simple and economical HPLC method using readily available reagents for the identification and quantification of hydrocortisone in pharmaceutical preparations would be an advantage in resource-poor countries.
The upsurge of autoimmune diseases such as adrenal insufficiency is a major health concern and the long-term use of conventional hydrocortisone tablets in the management of such a condition is problematic. The twice or thrice daily dosing of conventional hydrocortisone tablets to patients with adrenal insufficiency disease is incapable of mimicking the unique diurnal cortisol circadian pattern. Thus, most persons with adrenal insufficiency continue to suffer from poor therapeutic management resulting in poor quality of life and increased mortality. There is therefore the need for the development of creative and innovative treatment models for hydrocortisone replacement therapy in such dire situations. The use of controlled-release hydrocortisone oral dosage forms holds great promise with the capability to replicate the unique physiological pattern of hydrocortisone. In addition, such formulations are better able to manage and control the levels of morning androgen levels. The accurate monitoring of the quality of these promising new drug therapies is an important prerequisite to obtain quality healthcare [14]. The improved therapy will enhance patient compliance and ensure the delivery of controlled amounts of hydrocortisone at the absorption site compared to the immediate release pattern of conventional tablets [15].
The aim of this study was to develop and validate a simple, sensitive, and reproducible isocratic reverse phase HPLC method with ultraviolet/visible (UV) detection capable of determination and quantification of hydrocortisone in conventional and controlled-release pharmaceutical preparations. The application of the method in determining the content of hydrocortisone in controlled-release and commercially available conventional dosage forms was also studied.
Materials and Methods
Standards and Reagents
The reference material of hydrocortisone (Sigma Aldrich, USA), HPLC grade methanol (Fisher Scientific, UK), glacial acetic acid (Fluka, Germany), and water (double distilled) were used. All stock and working solutions were freshly prepared with distilled water for HPLC analyses.
Hydrocortisone Pharmaceutical Preparations
Fifteen commercially available hydrocortisone pharmaceutical preparations, comprising nine tablet and six injectable formulations, were sampled from retail pharmacies within the Kumasi and Accra Metropolitan areas of Ghana. Additionally, four controlled-release hydrocortisone tablet formulations, two controlled-release capsule formulations, and one conventional release hydrocortisone tablet formulation were obtained from the Department of Pharmaceutics and Microbiology, University of Ghana School of Pharmacy, Accra, Ghana.
Instrumentation and Optimized Chromatographic Conditions
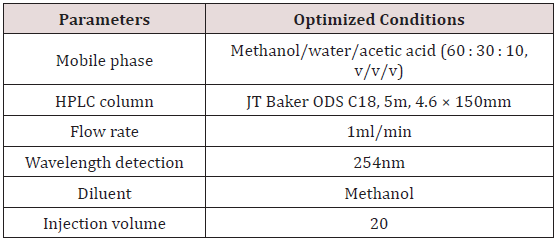
A chromatograph comprising a P100 Spectra Series pump with a 785A Programmable Perkin Elmer UV/VIS absorbance detector was employed in the study. Isocratic mode of elution was employed. Chromatographic separation was successfully achieved on a JT Baker® ODS C18 column (150 × 4.60 mm ID, 5 μm particle size). Ultraviolet detection at a wavelength of 254 nm was able to ensure good resolution of peaks. The injection volume was 20 μL and all analyses were undertaken at ambient temperature. A mobile phase consisting of methanol/water/acetic acid (60 : 30 : 10; v/v/v) at a flow rate of 1.00 ml/min was employed. All chromatographic data were acquired using PowerChrom series 280 integrator software.
Preparation of Standard Solution
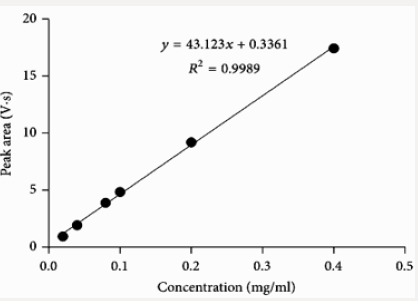
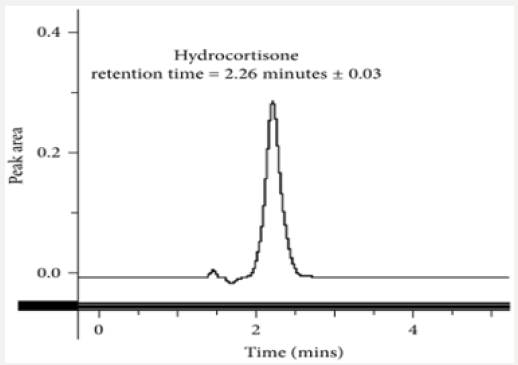
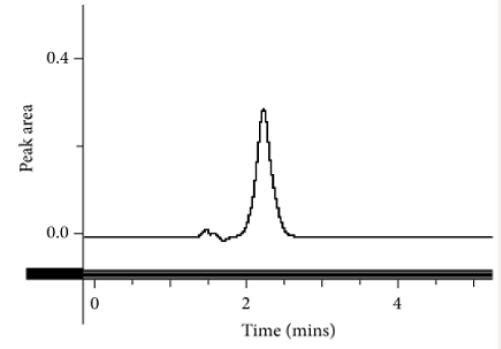
Fifty milligrams of hydrocortisone reference standard were accurately weighed and dissolved in 25 ml of methanol as the diluent. The solution was transferred into a 50 ml dry volumetric flask and was then sonicated for 5 min. The volume was made up to the mark with methanol and mixed thoroughly (1 mg/ml stock solution). A one in ten (1 in 10) dilution was made to obtain a working solution with concentration of 0.10 mg/ml of hydrocortisone. This solution with the required working concentration was then injected to determine the retention time of the analyte of interest using the developed method.
Analytical Method Validation
The new HPLC method was validated in terms of linearity, limits of detection and quantification, accuracy, precision (intraday and interday), specificity, robustness, and stability, in compliance with International Conference on Harmonization (ICH) guidelines [16].
Limits of Detection and Quantification: The limit of detection (LOD) and limit of quantification (LOQ) of the new HPLC method were determined from the linear regression curve obtained from the linearity of hydrocortisone. The slope and standard deviations of the responses of the linear curve were used. The formulae for the calculation of the LOD and LOQ are given below: where δ is standard deviation of the response; is slope of the calibration curve.
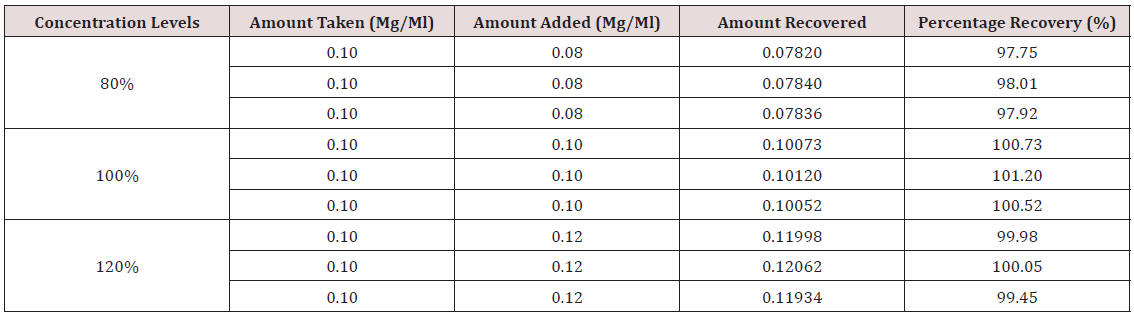
Accuracy: Into three separate 10 ml volumetric flasks 1 ml of a 1 mg/ml standard solution of hydrocortisone was pipetted. 0.8 ml, 1.0 ml, and 1.2 ml of formulated hydrocortisone solution (0.1 mg/ml) were added to the three volumetric flasks, respectively, to obtain concentrations at 80%, 100%, and 120% levels. The solutions were analyzed using the developed method. Triplicate determinations were made for each solution and mean percentage recovery calculated.
Precision: Both intraday and interday precision of the HPLC method were assessed. For intraday precision, three solutions of different concentrations were prepared in separate 20 ml volumetric flasks from the stock solution of hydrocortisone reference standard (1 mg/ml) by pipetting 8.0 ml, 2.0 ml, and 0.4 ml of the stock solution. Solutions were made up to volume with methanol. The various solutions where analyzed thrice during a particular day to obtain chromatograms from which recoveries and relative standard deviations (RSD) were calculated. For interday precision, a one in ten (1 in 10) dilution of the stock standard solution was prepared on three different occasions and analyzed. The solutions were analyzed on three consecutive days using the developed HPLC method to obtain chromatograms and the hydrocortisone content and relative standard deviation (RSD) were determined.
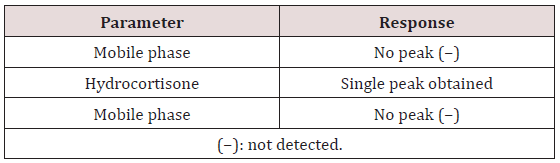
Specificity: The developed HPLC method was investigated for specificity to ensure there was no or minimal interference of analyte of interest from the solvent system. The mobile phase (blank) was analyzed, followed by an injection of a sample solution of hydrocortisone. After a ten-minute wash period, the blank was analyzed again.
Robustness: The robustness of the method was examined by introducing small changes in the composition of the mobile phase. Variation by ±10% was done with respect to the methanol component of the mobile phase while keeping the composition of the others constant. Chromatograms obtained were analyzed using the RSD of the responses obtained.
Stability: The stability of solutions of hydrocortisone were studied over a period of 8 hours. Triplicate injections were made each hour and the chromatograms recorded.
Assay of Hydrocortisone Preparations
An amount of finely powdered hydrocortisone powder from each of the nine commercial conventional tablet formulations equivalent to 2.5 mg hydrocortisone was individually weighed accurately and transferred to a 25 ml volumetric flask containing 10 ml of methanol. The solution was sonicated for 5 min and made up to volume with methanol. The resulting solution was then filtered using Whatman filter paper number 41. The filtrate obtained was analyzed by making triplicate injections. The controlled-release hydrocortisone tablet and capsule formulations were analyzed in a similar way. In the analysis of the hydrocortisone injections, aliquots equivalent to 2.5 mg hydrocortisone were pipetted from the six (6) injectable preparations into 25 ml volumetric flasks and treated similarly to above. The peak areas of sample were determined, and the amount of hydrocortisone was estimated from the linear regression calibration curves using the developed method. The official United States Pharmacopoeia method [11] was employed in analyzing the commercial samples. Statistical comparison between the official and developed methods was done using Student’s -test.
Results and Discussion
Controlled-release hydrocortisone preparations remain formulations of choice in the therapeutic management of adrenal insufficiency disease. This disease condition is a potentially lifethreatening autoimmune disorder that requires prompt diagnosis and management to avoid fatality. The use of controlled-release formulations results in more stable cortisol concentrations during the diurnal cortisol circadian pattern than conventional hydrocortisone products [17]. It is however essential that suitable and accurate analytical methods are made available to assess the quality of the products with regard to their content. The development and validation of a simple isocratic RP-HPLC method for the determination of hydrocortisone in both conventional and controlled-release pharmaceutical formulations were the focus of this study. Review of literature indicates the employment of normal phase stationary support material [18,19] and reverse phase stationary support material [20] for the development of HPLC method and further analyses of hydrocortisone products. There is yet no reported reverse phase HPLC with UV detection method capable of analyzing hydrocortisone in both conventional and controlled-release pharmaceutical formulations. The inclusion of acetic acid as a modifier in the mobile phase system ensured efficient resolution of hydrocortisone with minimal matrix effect from both conventional and controlled-release formulations. Readily available and cost-effective solvents employed in the mobile phase ensured an efficient resolution and separation of hydrocortisone on a reverse phase column. Table 1 presents the optimized chromatographic conditions employed in the determination of hydrocortisone.
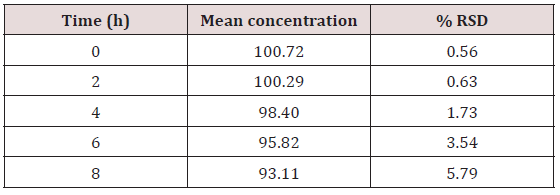
This method provides an option for the analyses of hydrocortisone in various salt forms compared to the individual methods available in some official compendia such as the United States Pharmacopoeia [11]. The analytical method being reported gave good linear detectable responses in the range of 0.02 to 0.4 mg/ml with coefficient of correlation of 0.9989 with wellresolved hydrocortisone and excipient peaks (Figures 2-5). The limit of detection and limit of quantification of 1.0662 × 10−2 mg/ml and 3.23076 × 10−2 mg/ml, respectively, obtained are an indication of the sensitivity of the method. The method showed good accuracy with good recoveries in the range of 98 to 101% recorded at three concentration levels of 80%, 100%, and 120% (Table 2). The method was reproducible with good intraday and interday precision of less than 1% RSD (Tables 3 & 4). The new HPLC method showed high specificity and the robustness was less than 1% RSD (Tables 5 & 6). The method also demonstrated good stability over a period of 8 h (<6% RSD) (Table 7).
Table 3: Intraday precision of proposed HPLC method showing the mean percentage recovery and RSD of hydrocortisone at different concentrations.
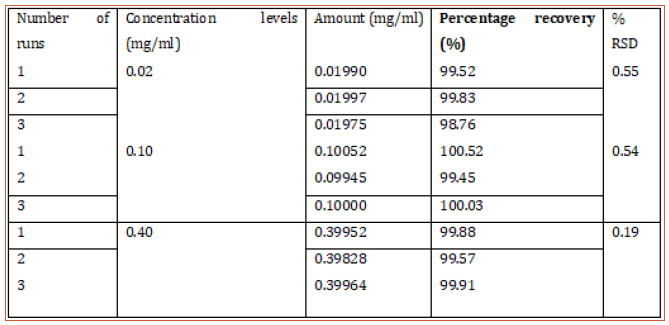
Table 6: Robustness of proposed HPLC method showing the mean percentage content of hydrocortisone when conditions were varied.
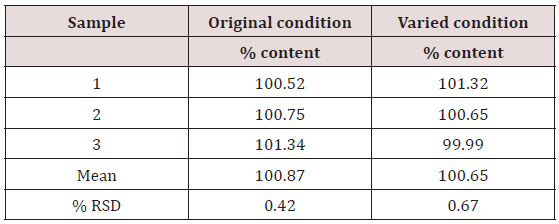
Table 7: Stability of HPLC method showing the mean recoveries of hydrocortisone in solution over an eight-hour period.
Application of Developed Method to the Analysis of Commercial Samples
Table 8: characteristics and labelled strength of commercial hydrocortisone preparations analysed using the developed HPLC method (DM) and the USP method.
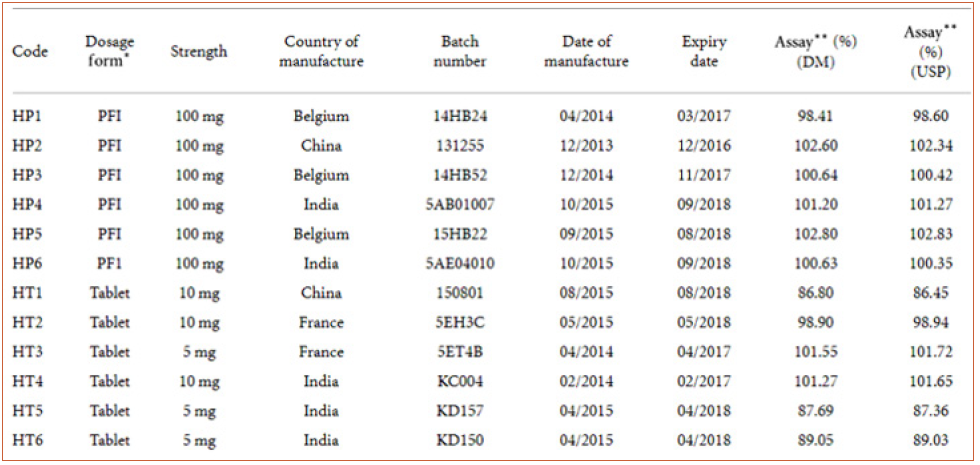
Table 9: Characteristics andsss labelled strength of pectin -based modified release hydrocortisone preparations analysed using the developed HPLC method.
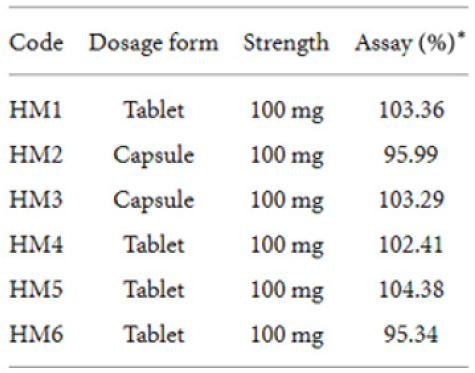
Table 8 presents the characteristics, labelled strength, and assay results of conventional hydrocortisone preparations which were analyzed using both the developed HPLC method and the official USP method. Statistical analysis (-test) of data from the two methods (official/USP and developed) showed no significant difference () at a 95% confidence interval (Table 9). The analytical method developed provides a relatively cheaper alternative technique for routine postmarket analyses of hydrocortisone formulations in resource-poor countries such as Ghana. The validated method was also successfully applied to the analysis of six controlled-release hydrocortisone tablet and capsule formulationsf. The application of the method in the analyses of tablets (conventional and controlledrelease) and parenteral formulations indicated the versatility of the analytical procedure. The analyses of the hydrocortisone formulations yielded percentage hydrocortisone content of 95- 104%, 96-103%, and 87-103% for the four controlled-release tablet formulations, two controlled-release capsule formulations, and fifteen conventional hydrocortisone formulations, respectively. All the controlled-release products were of good quality in terms of their content (percentage of content ≥ 90%). The percentage of content of hydrocortisone powder was 100.44%. Twelve products (all 6 injections, 6 tablets) out of the 15 commercial hydrocortisone products analyzed were determined to contain the right amounts of hydrocortisone as per United States Pharmacopoeia (USP) method and the developed HPLC method [11]. This study did not cover other pharmacopoeia specifications such as disintegration and dissolution tests.
Conclusion
It can be concluded that the developed process of hydrocortisone, hydrocortisone sodiumsuccinate
a) Rearrangement leading from the 11--OH compound to the 11--keto compound via the intermediate halohydrin, is carried out under far more gentle conditions that those described in the European Patent No. 30,368, which presents an advantage both in the level of the reaction yield because the formation of secondary or degradation products is limited,
b) An advantage on the industrial level to the extent that the synthesis is more economical; The blocking in position 3 is remarkably selective in contrast to the known blockings by enol ethers and ketals which lead to mixtures of products blocked in positions 3 and 3,17; The blocking in the 3--position in the invention is very stable under the reaction conditions used whether they are acidic or basic, and its elimination during synthesis, notably by the action of iodine in a basic medium or iodine in catalytic quantity under gentle oxidizing medium, is very easy; Access to hydrocortisone is possible without going through a hydroxylation stage in the 11--position which is the case with known synthesis using androstenedione at the start. Accordingly, this improves the overall yield of the process.
c) Method for preparation of hydrogenation cortisone sodium succinate reaction temperature is low, fast, easy to produce during the reaction of other impurities, fewer side effects and was concentrated, high yield, where water and other solvents content is low, to lay the foundation for post-drying, hydrogenation of the hydrocortisone sodium succinate prepared in line with CP2005 and USP28 standards, for clinical use
d) Finally concluded that the developed and validated simple, isocratic, sensitive RP-HPLC method is useful for the determination and quantification of hydrocortisone in conventional and controlled-release pharmaceutical formulations. The method was successfully applied to commercial samples which indicate the ability of the analytical method to distinguish between good and poor-quality hydrocortisone products.
References
- Diane Clare Rigge, Martin Frederick Jones (2005) Shelf lives of aseptically prepared medicines - stability of hydrocortisone sodium succinate in PVC and non-PVC bags and in polypropylene syringes. Journal of Pharmaceutical and Biomedical Analysis 38(2): 332-336.
- P Christen, F Kloeti, B Gander (1990) Stability of prednisolone and prednisolone acetate in various vehicles used in semi-solid topical preparations. Journal of Clinical Pharmacy and Therapeutics 15(5): 325-329.
- Claus Larsen, Peter Kurtzhals, Marianne Johansen (1988) Kinetics of regeneration of metronidazole from hemiesters of maleic acid, succinic acid and glutaric acid in aqueous buffer, human plasma and pig liver homogenate. International Journal of Pharmaceutics 41(1-2): 121-129.
- Menard Szczebara F (2003) Total biosynthesis of hydrocortisone from a simple carbon source in yeast. Nature Biotechnol 21:143-149.
- (2016) Hydrocortisone Drugs com American Society of Health System Pharmacists.
- Becker Kenneth L (2001) Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins pp. 762.
- Hamilton Richart (2015) Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab Coat Edition. Jones & Bartlett Learning pp. 202.
- (2016) Hydrocortisone Pregnancy and Breastfeeding Warnings.
- Walker SR (2012) Trends and Changes in Drug Research and Development. Springer Science & Business Media pp. 109.
- Caldato Milena CF, Fernandes Vânia, T Kater (2004) One-year clinical evaluation of single morning dose prednisolone therapy for 21-hydroxylase deficiency. Arquivos Brasileiros de Endocrinologia e Metabologia 48(5): 705-712.
- J Elks (2014) The Dictionary of Drugs Chemical Data: Chemical Data, Structures and Bibliographies. Springer pp. 316.
- Karmas George (1968) J Org Chem 33(6): 2436-2440.
- Venton (1975) J of Med Chem 18(1): 9-16.
- J Gregory Reid (1990) Corticoids from 17-oxosteroids. Tetrahedron Letters 31(26): 3669-3672.
- Aher Nilkanth G (2005) Synthesis of Bile Acid Dimers Linked with 1,2,3-Triazole Ring at C-3, C-11, and C-24 Positions. Synlett 14: 2155- 2158.
- Breslow Ronald (1980) Biomimtic Control of Chemical Selectivity. Acc Chem Res 13(6): 170-177.
- Harburn James J (1998) Synthesis of Novel Steroidal Inhibitors of HIV-I Protease. Tetrahedron 54(39): 11907-11924.
- Peterson DH (1952) Microbiological Transformations of Steroids: I Introduction of Oxygen at Carbon-11 of Progeserone. J Am Chem Soc 74: 5933-5936.
- Salunke Deepak B (2006) Amino Functionalized Novel Cholic Acid Derivatives Induce HIV-1 Replication and Syncytia Formation in T Cells. J Med Chem 49(8): 2652-2655.
- Salunke Deepak B (2005) An efficient method for the synthesis of methyl 11α-amino-3α,7α-diacetoxy-12-oxo-5β-cholan-24-oate. Tetrahedron 61(14): 3605-3612.
- (2015) International Application No. PCT/IN2014/000730, International Search Report and Written Opinion dated, p. 8.
- Epstein D (2012) The effect of physiological concentrations of bile acids on the in vitro growth of Mycobacterium tuberculosis. South African Medical Journal 102(6): 522-524.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...