Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
Synthesis, 1H-NMR, GC-MS Characterization, and in Vitro Bactericidal Studies of an Aqueous Soluble Vanadium (IV) Complex with Dipicolinic Acid and Methylsalysilateligands Volume 1 - Issue 5
Tulio Chavez Gil*1,2, Amanda A Robinson1, Hany F Sobhi1, Alex Ramirez Gonzalez2, Valerie Madera Garcia2 and Jonathan Vega Estevez2
- 1Department of Natural Sciences, Coppin State University, USA
- 2Department of Biology, Chemistry, and Environmental Sciences, Inter American University of Puerto Rico, USA
Received:February 20, 2018; Published: February 23, 2018
Corresponding author:Tulio Chavez Gil, Department of Natural Sciences, Coppin State University, 2100 West North Ave, Baltimore, MD 21216 - U.S.A, USA
DOI: 10.32474/AOICS.2018.01.000124
Abstract
The fast emerging of superbugs’ resistance against common commercial antibiotics has been timely challenges and temporarily assuaging outbreak concerns in modern medicine. But coincidentally, there has been a significant retraction on new drugs design as effective antimicrobial medicines by the major pharmaceutical companies with the coinciding escalation in global nosocomial infection. Originally, antibiotics were described as “produced by microorganisms and which possess the property of inhibiting the growth and even of destroying other microorganisms" [1]. Now a day's most commercially antibiotics however, are obtained from other sources than microbiological processes with many clinically “antibiotic drugs” being either synthetic or semi-synthetic ones. Some of these new synthetic antibiotics are characterized by the presence of specific metals to function properly as an integral part of the structure and function as defined in the medicine SAR's interpretation [2]. Therefore, the removal of essential metal ions from these medicines might results in the deactivation and/or change in the structure of the mas observed on bacitracin, bleomycin (BLM), streptonigrin (SN), and albomycin [3]. In addition, the presence of metalsin some antibiotics engender bacteria with biochemical consequences but do not significantly affect the structure of the drug, such as occur in metallated tetracycline's (TCs), aureolic acids, and quinolones. Similar to the case of "metallo proteins,” these families of antibiotics are thus dubbed "metallo antibiotics” and are under scrutiny in our research with the aim of improve the reduced scope of non- β -lactamase bactericidal/ bacterio static agents.
To that, we are occupied in the chemical synthesis of artificial chelators and metal complexes obtained this time between dipicolonic acid (DPA), 2-methylsalycilate (MeSal), and methavanadate (V=O), for in vitro antibacterial screening and results compared with those obtained using commercially antibiotics. Here we report on the microbiology results performed with a vanadium VIV complex (vanadibacter) that is probing to have moderate to excellent bactericidal effects on a broad scope of gram-positive/gramnegative strains, especially against family of pathogenic superbugs which are considered the leading cause of nosocomial infections throughout the world and so called ESKAPE group. To that in vitro tests were carried out on Enterococcus faecalis, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter aerogenes, with those cells grown under iron uptake limitations [4].
Material and Methods
All microbiological standards and vanadium medium were prepared in ultra-pure water (UPW) and purified through a Barnstead/Thermolyne (Dubuque, IA) with a water resistance from 0.01 to 18.3 mega-ohm-cm (18-MΩ). The elemental analysis was checkedon an Agilent 7890GC system coupled to an Agilent 7000GC/MS Triple Quad spectrometer with an Elite-I fused silica capillary column (30mx0.25mm IDx0.25μm (HP-5ms). The proton NMR spectrum was collected on a Bruker Avance III 400MHz NMR spectrometer; FTIR spectra were collected as KBr disk on a Nicolet iS50 FTIR spectrometer. The strain microorganisms (ATCC), trypcase soy agar (TSA), and Muller-Hinton Agar (MHA) were purchased by either Carolina Biological Supply Command (Burlington, NC) or from B. D. biosciences. Inc (Sparks, MD). The commercial antibiotics novobiocin, penicillin, and streptomycin were purchased from Sigma Chemical Co (St. Louis, MO).
[VIV(DPA)(MeSal)O] (vanadibacter) complex synthesis
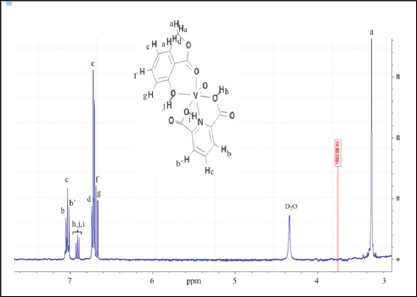
0.100g (0.60 mmol) of dipicolinic acid was mixed with 0.0701g (0.60mmol) of NH4VO3 in 5.0mL of THF previously degassed under dried argon (Ar). An excess of 0.2348g (1.54mmol) of 2-methyl salicylate were drop-wised and the reaction was keep out at room temperature for 12h under magnetic stirring. Single yellowish crystals were obtained, washed with cold ethanol:pentane mixture and dried under vacuum over fused CaCl2. Yield 0.185g (83.7%) (Scheme 1). FT-IR (KBr disk, cm-1): 3170 and 3088 DPA (OH carboxylic.),2924 2-Mesal (OHphenolic), 2865 v CH3,1678 (V-O=Csalicylate), 1429 v (V-N=), 1341 v (C=N),1114-1116 vassymetric(OH), 575(V=O). 1H-NMR (400MHz, δppm (D2O), (Figure 1): 7.05-7.04 (d,2HBB', JHH=0.01Hz), 7.03-7.04 (d, HC, JHH=0.01Hz), 7.01-7.00 (d,2HB,B, JHH=0.01Hz), 6.92-6.88 (t, (d,3HDpA, JHH=0.01Hz), 6.73 (s, 2HG), 6.72 (s, He), 6.60 (s, Hf), 6.68-6.66 (d, HD, JHH=0.01Hz), 1.82 (s, HA, 3H). Non-unpaired electrons, no transition metal with unpaired spins causing large shifts (tens and hundreds of ppm), or large number of line broadening are absente in the recorded spectra as proof of complex purity.
Figure 1: 400-MHz, 1H-NMR spectra of [VIV(DPA) (MeSal)O] in D2O at room temperature, with labeled protons at 294 K.
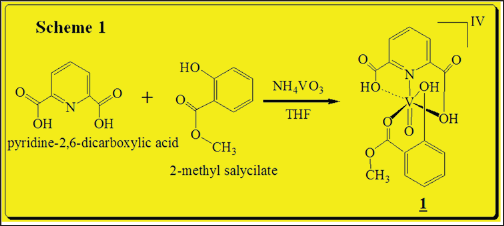
Scheme 1: Synthesis of Vanadium(IV) complex.
GC-MS
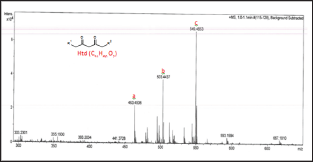
The samples were prepared by dissolving 5.0μg of complex in 20.0ml mixture of ethyl acetate: isopropanol 1:1(v/v) (HPLC grade) and filtered through a 0.2μm sterile syringe filters. An electron ionization system of 50eV was used for GC-MS determinations at scan intervals of 1.0 second and fragments from 45 to 450Da with GC total running time as 30 minutes. The spectral data was analyzed with the adopted software Turbo mass. Five major peaks were detected at retention times 12.19 min, 19.23 min, and 22.44 min (Figure 2). The peak at tR 22.44min displayed a molecular ion at m/z 386.1 suggesting a structural formula C15H13NO8V with calculated FW=386.2g/mol. Loss of a methyl (-CH3) from the molecule resulted in a m/z=371 mass for a C14H10NO8V+ fragment. Other reduction and fragmentations generate ions with decreased mass. Thus, asubsequent loss of a methoxy (+O-CH3) ionhas an m/z=355 which suggest a composition of C14H10NO7V+ ion. Anm/ z=293 suggest a square planar ion with chemical composition of C9H7NO4V+. The total loss of 2-MeSal ligand resulted in a molecular ion with m/z=207 suggesting a structural formula C7H9O4V+. Finally, the loss of a phenol ion (+HO- η 6-C6H6) generate a fragment with m/ z=280 and chemical ion composition C9H9NO7V+.
Figure 2: GC-MS spectra of [VIV-(DPA) (2-MeSal)O] inethyl acetate:isopropanol (1:1, v/v)mixture with the carrier gas (Ar) at constant flow rate of 2.0 mL/min and split ratio of (10:1), injector temperature of 250 oC; and ion-source final isothermal at 280 oC.
Results and Discussion
Chemistry and pharmacology
In vitro antibacterial screening and MIC determination: The objective of this research is to evaluate the antibacterial activity of a vanadate complex against cell lines that include but are not limited to [Enterobacter aerogenes (ATCC, 13048), Staphylococcus aureus (ATCC, 25.923), Klebsiella pneumoniae (ATCC, 13883), Acinetobacter baumannii (ATCC, 19606), Pseudomonas aeruginosa (ATCC-27853), Enterococcus. faecalis (ATCC, 51299)], and to compare with commercially antibiotics. The agar used was Mueller-Hinton II and the preparations of this medium per manufacturer specifications are 38 grams of the dehydrated medium in a liter of distilled water, which is heated in a stainless steel pot while agitating carefully with glass bar and let to boil for 1 minute. The prepared mediums pH should be 7.4±0.2 at 25°C that are then dispensed in 16 cm test tubes, 15mL per tube and placed in a rack to be autoclaved for 15 minutes at 121 °C and 15 psi. After the tubes were retrieved from the autoclave they were kept in a thermo stated bath at 50 °C and set there for 15 minutes. The content of the tubes were then poured on 100 mm Petri dishes and let stand for 15 minutes so the agar medium can solidify, followed by the bacteria inoculation with a sterile swab in a "Z” pattern on every dish and let stand inverted with the lid on for 1 hour to let the excess water drain and vaporize. The screening was carried out by well-diffusion disc method, which is interpreted as a qualitative to semi quantitative test. Briefly, the compound was tested against using Muller Hinton agar medium. The sterilized (autoclaved at 121°C for 15min) medium (40-50°C) was poured into Petri dishes to give a depth of 3-4mm and allowed to solidify in 15ml quantities of nutrient agar. The inoculums were prepared in the same medium with a density adjusted to a 0.5 McFarland turbidity standard [1.5x108 colony-forming units (CFU)/ml] and diluted in a ratio 1:10 for the broth micro dilution procedure. Practicing aseptic techniques the inoculation was performed near an open flame by using a Bunsen burner.
The suspensions of microorganism were then streaked on plates with 0.1mL of a 10-3 dilution of each bacterial culture. Paper discs (5mm in diameter) were impregnated with various concentrations of compound and placed on test organism-seeded plates. On each quadrant of Petri dish was placed a thin wafer containing different antibiotic (streptomycin, penicillin, novobiocin, and [VIV(DPA) (Mesal)O] and pre-incubated for 1.0 hour at room temperature and finally incubated at 37° for 24h. A blank disc impregnated with solvents followed by during off was used as negative control. The minimum inhibitory concentration (MIC) tests for the VIV complex were determined by micro dilution techniques according to NCCLS (2000) procedures [5]. To it, two susceptibility endpoints were recorded for each isolated which is defined as the lowest concentration of compound at which the microorganism tested did not demonstrate visible growth zone [6]. The diameters of zone of inhibition produced by both commercial antibiotics and vanadium complex (Figure 3) were then compared with the standard antibiotic streptomycin (30μg/disc), which possess the highest antibacterial activity from all the commercially tested antibiotics. Each sample was tested in triplicate for the determination of antibacterial activity. The MIC values determinate with vanadate complex were aureus), 2.8 (P. aeruginosa), 1.0 (E. faecalis), respectively, and are 2.0 (A. baumannii), 2.5 (E. aerogenes), 2.5 (K. pneumoniae), 1.5 (S. reported in Table 1.
Figure 3: Antimicrobial screening depicting antibiotics inhibition zone for st=streptomydn, no=novobiocin, p=penicillin and v=vanadium complex, against the ESKAPE nosocomial family using the well-diffusion disc method.
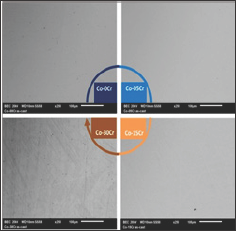
Table 1: Well-diffusionand minimum inhibition concentration (MIC)values of aqueous soluble [VIV(DPA)(MeSal)O]1 complex, compared with commercial antibiotics (see Figure 3).
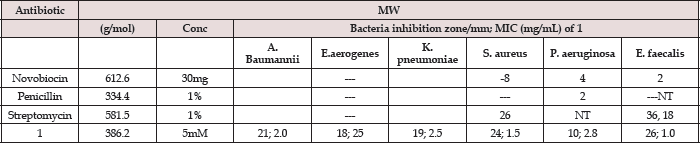
MW= molecular weight, Conc = concentration of antimicrobial, mM = millimolar, mm = millimeter, μ g = microgram, mL = milliliter, — = inhibition 2< mm, NT = not tested.
Note: The control disc used for solvent had no zone of inhibition, so this data was omitted for analysisand only the representative data in the form of mean of three tests ± SE were considered for analysis/interpretation.
Conclusion
The structure of [VIV(DPA)(MeSal)O] as a bioinorganic complex is based essentially on itsphysical chemistry 1H-NMR, GC-MS, and FTIR characterization. On these bases, the structureof this antibacterial inorganic complex is proposed such that steric constraints and hydrophobic properties are eliminated. Furthermore, the 2-methyl salicylate ligand is able to form a stable molecular binding in the oxovanadium complexto act as an absolutely water-soluble moiety forantimicrobial assays. The complexshowed good to strong antibacterial activity againstpathogenic superbugs such as Staphylococcus aureus, Streptococcus faecalis, Klebsiella pneumoniae, Enterobacter aerogenes, Pseudomonas aeruginosa, and Acinetobacter baumannii. The preliminary antimicrobial screenings with this aqueous soluble oxovanadium complex shows results better than that obtained with commercially novobiocin and penicillinantibiotics up to70-85% to control pathogenic b-lactamase resistant bacteria.
Acknowledgement
The authors (T. Ch-G, AA. R, HF. S) is grateful to Coppin State University for administrative help and the University System of Maryland for 400MHz NMR, and GC-MS spectrometers acquisition. Special thanks are expressed (In memoriam) to Prof. Ma Y Perez (Director, Ronald E. McNair program-CFDA No. 84.217A grant- IAUPR-SG) for research financial support and for an under graduate research scholarship (T Ch-G, J.V-E).
References
- D Rehder (2008) Bioinorganic Vanadium Chemistry. (1st edn), John Wiley & Sons TextBooks, UK.
- Ge R, Sun H (2007) Bioinorganic Chemistry of Bismuth and Antimony: Target Sites of Metallodrugs. Acc Chem Res 40(4): 267-274.
- Soory M (2008) A Role for Non-Antimicrobial Actions of Tetracyclines in Combating Oxidative Stress in Periodontal and Metabolic Diseases: A Literature Review. Open Dent J 2: 5-12.
- C Baysse, D De Vos, Y Naudet, A Vandermonde, U Ochsner (2000) Vanadium interferes with siderophore mediated iron uptake in Pseudomonas aeruginosa. Microbiology 146: 2425-2434.
- Lyalikova NN, Yukova NA (1992) Role of microorganisms in vanadium concentration and dispersion. Journal of Geomicrobiology 10(1): 15-25.
- Kiehlbauch JA, Hannett GE, Salfinger M, Archinal W, Monserrat C (2000) Use of the National Committee for Clinical Laboratory Standards Guidelines for Disk Diffusion Susceptibility Testing in New York State Laboratories. J Clin Microbiol 38(9): 3341-3348.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...