Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Review Article(ISSN: 2637-4609) 
Experimental Approach, Computational DFT Investigation and a Biological Activity in the Study of an Organic Heterocyclic Compound Volume 4 - Issue 1
Tribak Z*
- Laboratory of Applied Chemistry, Sidi Mohamed Ben Abdellah University, Morocco
Received: January 10, 2019; Published: January 24, 2019
*Corresponding author:Tribak Z, Laboratory of Applied Chemistry, Faculty of Sciences and Technology of Fez, Sidi Mohamed Ben Abdellah University, B.P. 2202-Road Imouzzer Fez Morocco
DOI: 10.32474/AOICS.2019.03.000177
Abstract
The title compound TZ1 was synthesized by N-alkylation reaction, and its structure was confirmed by 1H NMR, 13C NMR and IR, it was screened for their in vitro antibacterial activity by the agar well diffusion method against four bacteria, Gram-positive (Bacillus cereus, Staphylococcus aureus) and Gram-negative (Escherichia coli, Pseudomonas aeruginosa). The molecule was studied with the density functional theory (DFT) at B3LYP/6–31G (d,p) level in order to determine the relationship between the molecular structure and the antibacterial inhibition behavior. The molecular geometry, frontier molecular orbitals and Mulliken atomic charge of the compound are investigated to get a better insight of the molecular properties. The molecular electrostatic potential (MEP) for a compound was determined to check their electrophilic or nucleophilic reactivity. The theoretical parameters offer significant assistance to understand the antibacterial inhibition mechanism indicated by the molecule and are in full agreement with the experimental results.
Keywords:5-Chlorosatin derivatives; N-alkylation reaction; antibacterial activity; DFT; Molecular electrostatic potential
Graphical abstract:
Introduction
The 5-Chloroisatin is well documented as an important heterocyclic compound in the field of medicinal chemistry. My recently published book and review [1,2] contain a special chapter, dedicated to the chemistry of 5-Chloroisatin and their derivatives.
The 5-chloro-1H-indole-2 3-dione structure is a heterocyclic compound which easily participates in chemical reactions. Its bonding sites are analogous to pyrrole. As shown in Scheme 1, 5-Chloroisatin is reactive at four different positions including the carbon atom 3, nitrogen atom 1, the C2–C3 p-bond and the C2–N sigma bond.
This moiety of 5-Chloroisatin and their derivatives possess pharmacological and chemotherapeutic properties such as anti- cancer [3], anti-diabetic [4], anti-inflammatory [5], anti-malarial [6], anti-bacterial [7], anti-fungal [8], anti-viral [9] and others drugs for treatment of several diseases [10].
Density functional theory (DFT) has become a convenient method to decipher experimental results, in antibacterial activity; this technique makes it possible to accurately predict the inhibition efficiency of organic compounds on the basis of electronic and molecular properties as well as reactivity indexes [11]. The objective of current study is to explore relationship amongst structure and electronic properties of the synthesized 5-chloro-1- (2- (dimethylamino) ethyl) indoline-2,3-dione (TZ1) using DFT. Then, the evaluation of its antibacterial activity. (Scheme1)
Experimental Details
Chemistry
Melting points were determined using the kofler bench apparatus and uncorrected. The spectra of 1H NMR spectra were recorded in CDCl3 on the Brucker Avance-300 spectrometer, operating at 300MHz and at 75MHz for 13C-NMR using TMS as an internal standard and Spin resonances are reported as chemical shifts (d) in parts per million (ppm). Infrared Spectra were run on AVATAR 320 AEK0200713 spectrometer and frequencies are reported in cm-1. The purity of the synthesized compound was confirmed by thin layer chromatography (TLC), performed on Silica gel 60 coated plates. UV light was used for the visualization of TLC spots [12].
General procedure
The 5-Chloroisatin derivatives TZ1 was prepared by mixing 0.2g (1.1 mmol) of 5-chloro-1H-indole-2,3-dione, (0.23g, 1.16mmol) of potassium carbonate in 15mL of N-N dimethylformamide (DMF) and (0.035g, 0.10mmol) of BTBA, then, the reagent is slowly added, the mixture is left at room temperature for 48 hours. The reaction mixture was concentrated by using rota vapor. The solid was separated out by filtration. It was carefully checked by thin layer chromatography. The compound was isolated by column chromatography by using different fractions of n-hexane and ethyl acetate [13-16].
Yield=89%; mp: 114 °C ; 1H NMR (CDCl3) δppm 7.53-7.54 (m, H, HAr); 7.51 (d, H, HAr, J=9Hz); 6.90 (d, H, HAr, J=9Hz); 3.85 (t, 2H, CH2, J=9Hz); 3.75 (t, 2H, CH2, J=9Hz); 2.15 (m, 6H, CH3). 13C NMR (CDCl3) δppm: 184.59 (C=O); 164.45 (N-C=O); 146.22, 141.13, 110.39 (Cq); 138.59, 126.08, 113.36 (CHAr); 55.90, 46.79 (CH2); 45.09 (CH3). Infra Red (KBr) cm-1: 3565, 3174, 30815 (C-H), 2975, 1720 (C=O), 1607 (NC=O), 1445, 1472 (C=C) 1185,1123 (N-C), 654 (C-Cl).
Antibacterial screening
Synthesized compound TZ1 was screened for their antibacterial activity against two Gram positive (Bacillus cereus and Staphylococcus aureus) and two Gram negative (Escherichia coli and Pseudomonas aeruginosa) bacteria by the agar well diffusion method, using LB medium (Luria Bertani medium: yeast extract 5.0g, peptone 10.0g, sodium chloride 5.0g, distilled water 1000mL). This technique was recommended by CLSI [17].
A sterile paper disk was placed on the surface of each plate and impregnated with 5μL of the TZ1 solution at a final concentration of 10mg/mL. Then, the plates were incubated at 4 °C for 2 hours to permit good diffusion before incubation at 37±2 °C for 24 hours. The diameters of the inhibition zones were measured in mm with the caliper. A disc impregnated with 2% dimethylsulfoxide as a negative control was made the experiment was carried out in triplicate.
In order to determine the Minimum inhibitory concentration (MIC) values, we started by the dilution of the TZ1 was prepared in a Mueller Hinton broth supplemented with bacteriological agar, to reach a final concentration between 5mg/mL and 0.004mg/ mL, 50μL of bacterial inoculum was added to each well at a final concentration of 106CFU/mL. DMSO (2%) was used as a negative control. The final concentration of our product was between 5mg mL-1 (3rd well) and 0.019mg mL-1 (well 11). Plates were incubated at 37 °C for 24 hours. After 2 hours of a subsequent incubation, bacterial growth was revealed by reduction of blue dye resazurin to pink resorufin [18].
Including, the minimum bactericidal concentrations (MBC) which is the last step in the protocol, a bactericidal control is carried out 24 hours earlier by streaking on a platelet agar, after microdilution to the broth by spreading 5μL of the negative wells on Luria Bertani agar plates.
Theoretical calculations
The computational studies of compound TZ1 were performed
using the GAUSSIAN 09W [19] program package and visualized
with the Gauss View on a personal computer using density
functional theory (DFT) method with 6−31G (d,p) as the basis set
[20]. The using HOMO and LUMO orbital energies, the ionization
energy and electron affinity can be expressed as: IP = -EHOMO, EA =
-ELUMO, respectively. The total hardness, η and electronegativity χ
were given by the following relations:
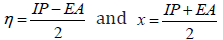 [21]. These DFT molecular parameters, and the equations used for
their calculations are presented in the Table 1.
[21]. These DFT molecular parameters, and the equations used for
their calculations are presented in the Table 1.
Result and Discussion
Synthesis of 5-chloro-1-(2-(dimethylamino) ethyl) indoline- 2,3-dione (TZ1) is outlined in Scheme 2, it was prepared according to a similar previous procedure [22]. 5-chloroisatin was used as a starting material for the synthesis of various substituted indole derivatives [23-27].
The (TZ1) was synthesized by the N-alkylation reaction of 5-chloro-1H-indole-2,3-dione in DMF, a base K2CO3 and a TBAB catalyst was added to a stirred solution at room temperature, Chloro-N,N-dimethylethanamine was added dropwise to the mixture under conditions of catalysis by phase transfer for 48 hours, the reaction was monitored by thin layer chromatography. After this time, the mixture was filtered and concentrated and dried under vacuum to afford the required product. The complex was obtained in good yield, stable in air, and is colored solid, soluble in methanol, chloroform, DMF, and DMSO.
The 1H-NMR, 13C-NMR and IR were used to assign the structure of synthesized compound. (Scheme 2)
Antibacterial activity
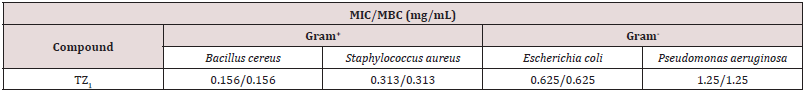
The In vitro antimicrobial screening tests of synthesized compound TZ1 was carried out as an antibacterial activity. the tested compound showed biological activity against different types of Gram-positive (Bacillus cereus and Staphylococcus aureus) and Gram negative bacteria (Pseudomonas aeruginosa, Escherichia coli), it showed zones of inhibition of MIC/MBC values ranging from 0.156/0.156 to 0.313/0.313mm against the Gram-positive bacteria and between 0.625/0.625 and 1.25/1.25mm against Gram-negative bacteria.
Coordination enhances the antibacterial activity and the TZ1 in the present study are more active against Gram-positive than Gram-negative bacteria [28]. On the other hand, it should also be noted that the presence of nitrogen and oxygen atoms which are the highest values of the negative charge on the molecule TZ1 suggesting that these centers have the maximum electron density and would preferentially interact with the micro-organisms Gram positive then increases the antibacterial potential.
Computational details
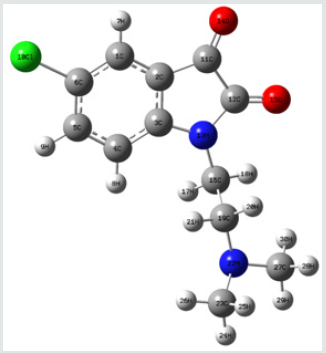
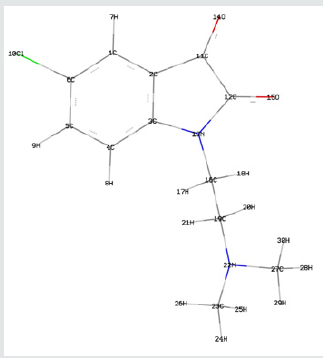
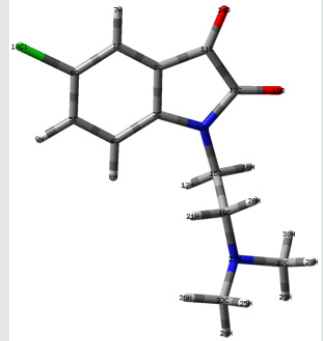
Frontier orbital energy analysis and other global reactivity descriptors: The all optimized structures along with the numbering scheme of TZ1 at DFT/B3LYP level using the 6-31+G (d,p) basis are shown in Figures 1-3.
The HOMO-LUMO orbitals help to characterize the chemical reactivity and kinetic stability of the molecule.
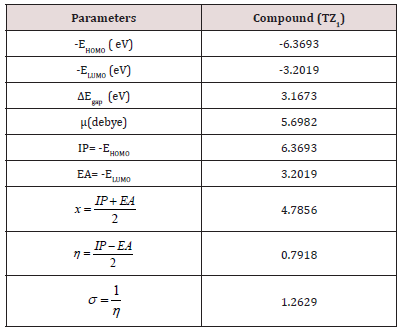
The analysis of the HOMO highlights the areas of the molecule that can donate electrons to electrophilic species while the analysis of the LUMO predicts the regions of the molecule with high affinity to accept electrons from nucleophilic species. The calculated HOMO–LUMO energy gap value is found to be 3.1673 eV (Table 2).
A molecule with a small frontier orbital gap (ΔEgap =3.1673 eV) is generally associated with a high chemical reactivity, low kinetic stability and is also termed as soft molecule [29]. The 3D plots of the frontier orbitals HOMO, LUMO for the TZ1 are shown in Figures 4 & 5.
The dipole moment (μ(debye= 5.6982) tells about the polarity of the molecule. The higher value of dipole moment in case of TZ1 molecule is mainly attributed to an overall imbalance in charge from one side of a molecule to the other side is also evident from the MESP plot. DFT calculation gives an idea about the substance reactivity and site selectivity of the frameworks. By the computed value of HOMO and LUMO energy values for the TZ1, the electronegativity (χ), total hardness (η), Softness (σ), can be calculated. The significance of (η) and (σ) is to evaluate both the reactivity and stability [31].
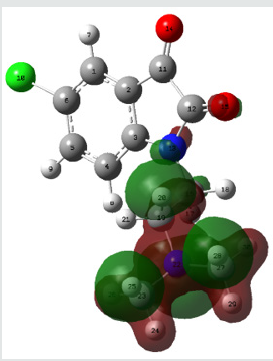
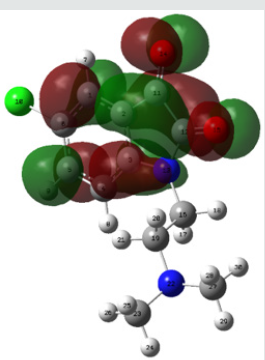
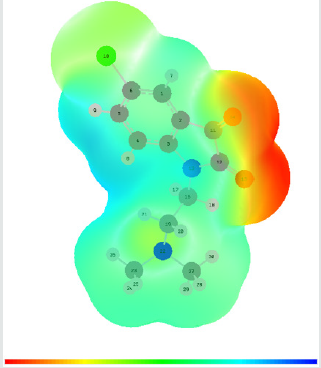
Molecular electrostatic potential (esp) map: The molecular electrostatic potential mapped surfaces show the charge distributions of molecules three dimensionally which give clear and special signature of the interactions of the compounds [31].
The molecular electrostatic potential is related to the electronic density and a very useful descriptor for determining sites for electrophilic attack and nucleophilic reactions as well as hydrogenbonding interactions [32].
The MEP mapped surface of the compound TZ1 was calculated by DFT/B3LYP at 6 31G (d,p) basis set and MEP surface are plotted in Figure 6. Red, blue and green colors represent regions of the most electro negative, most electro positive electrostatic and zero potential, respectively [33].
Indeed, it is clear that in the compound TZ1 oxygen atom (red region) and nitro groups react with electrophilic sites, in all hydrogens atoms (blue region) react with nucleophilic sites.
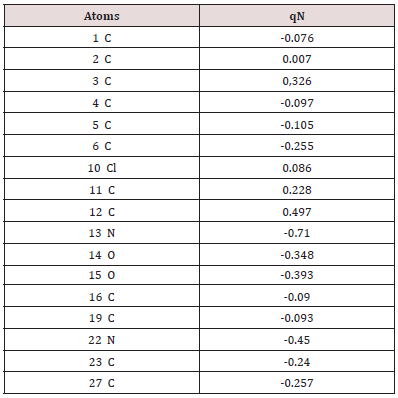
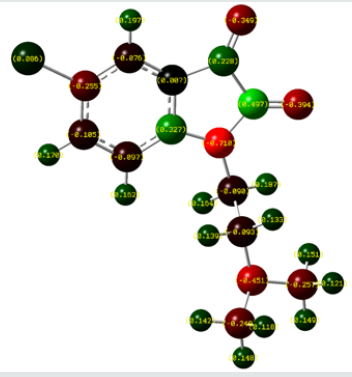
Mulliken charges analysis
The Mulliken atomic charges have a significant role in the application of quantum chemical calculations to molecular systems, by determining the electron population of each atom as defined by the basis function [34]. Table 3 exhibits the calculated mulliken atomic charges except for atoms H by DFT/B3LYP at 6 31G (d,p) basis set. Also, the color range in the scale of positive and negative charge and graphical representation for Mulliken atomic charges of TZ1 is shown in Figure 7.
From the listed tabulated values (Table 3) of atomic charge, we can summary that the charge on the carbon atom (C6) is greater than other carbon atoms in the all compounds because it is connected to electronegative chloride atom (Cl10). Then, all nitrogen and oxygen atoms (N13, N22, O14 and O15) are the most negatively charged ones, suggesting that these centers have the maximum electron density, which can interact with the positively charged part of the receptor easily.
Conclusion
In summary, we report the synthesis and characterization of 5-chloro-1- (2- (dimethylamino) ethyl) indoline-2,3-dione (TZ1) in excellent yield. The antibacterial activity of TZ1 has been explored experimentally and by quantum calculations. The frontier orbital energy analysis, mulliken atomic charges and electrostatic potential were also studied by using the DFT method at B3LYP/6–31G (d,p). The antibacterial bioassay showed that it possessed excellent activity.
Acknowledgements
The author would like to thank all the people who helped to carry out this work such as 1H NMR, 13C NMR, IR, and for antibacterial activity.
References
- Tribak Z, Skalli KSO (2017) Synthèse de nouveaux dérivés de 5-Chloroisatine. Univ Europ p. 1-60.
- Tribak Z, Skalli MK, Senhaji O, Rodi YK, Haoudi A, et al. A Review on Recent Advances and Applications of 5-Chloroisatin and its Derivatives in Design and Synthesis of New Organic Compounds. pp. 41–50.
- Bettayeb K, Tirado OM, Marionneau-Lambot S, Ferandin Y, Lozach O, et al. (2007) Meriolins, a new class of cell death-inducing kinase inhibitors with enhanced selectivity for cyclin-dependent kinases. Cancer Res 67(17): 8325-8334.
- Dropinski JF, Akiyama T, Einstein M, Habulihaz B, Doebber T, et al. (2005) Synthesis and biological activities of novel aryl indole-2-carboxylic acid analogs as PPARγ partial agonists. Bioorganic Med Chem Lett 15(22): 5035-5038.
- Pergola C, Dodt G, Rossi A, Neunhoeffer E, Lawrenz B, et al. (2008) ERK-mediated regulation of leukotriene biosynthesis by androgens: A molecular basis for gender differences in inflammation and asthma. Proc Natl Acad Sci 105(50): 19881-19886.
- Agarwal A, Srivastava K, Puri SK, Chauhan PMS (2005) Synthesis of substituted indole derivatives as a new class of antimalarial agents. Bioorganic Med Chem Lett 15(12): 3133-3136.
- Yamamoto Y, Kurazono M (2007) A new class of anti-MRSA and anti-VRE agents: Preparation and antibacterial activities of indole-containing compounds. Bioorganic Med Chem Lett 17(6): 1626-1628.
- Williams JD, Drach JC, Townsend LB (2005) Synthesis and antiviral activity of some 2-substituted 3-formyl- and 3-cyano-5,6-dichloroindole nucleosides. Nucleosides Nucleotides Nucleic Acids 24(10-12): 1613- 1626.
- Korba BE, Gerin JL (1995) Antisense oligonucleotides are effective inhibitors of hepatitis B virus replication in vitro. Antiviral Res 28(3): 225-242.
- Nosova EV, Lipunova GN, Charushin VN, Chupakhin ON (2018) Fluorinecontaining indoles: Synthesis and biological activity. J Fluor Chem 212: 51-106.
- Gosav S, Paduraru N, Maftei D, Birsa ML, Praisler M (2017) Quantum chemical study of a derivative of 3-substituted dithiocarbamic flavanone. Spectrochim Acta - Part A Mol Biomol Spectrosc 172:115-125.
- Tribak Z, Haoudi A, Kandri Rodi Y, Elmsellem H, Skalli MK, et al. (2016) Synthesis and reactivity of new heterocyclic systems derived from 5-chloro- 1H-indole-2,3-dione. Moroccan J Chem 4(4):1157-1163.
- Tribak Z, Kandri Rodi Y, Haoudi A, Essassi EM, Capetc F, et al. (2016) 1-(12-Bromododecyl)-5-chloroindoline-2,3-dione. IUCrData 1: x160971.
- Tribak Z, Kandri Rodi Y, Haoudi A, Essassi EM, Capetc F, et al. (2016) 1-Allyl-5-chloroindoline-2,3-dione. IUCrData 1: x160862.
- Tribak Z, Kandri Rodi Y, Haoudi A, Essassi EM, Capetc F, et al. (2016) 1-Benzyl-5-chloroindoline-2,3-dione. IUCrData 1: x160854.
- Zineb Tribak, Youssef Kandri Rodi, Amal Haoudi, El Mokhtar Essassi F, Zouihrid, et al. (2016) 5-Chloro-1-methylindoline-2,3-dione. IUCrData 1: x160913.
- Tribak Z, Amin OEl, Skalli MK, Senhaji O, Rodi YK, Iraqui MH (2017) Synthesis, Characterization, and Antibacterial Activity of Some Novel 5-Chloroisatin Derivatives. Int J Eng Res Appl 7(6): 66-70.
- Tribak Z, Skalli M, Haoudi A, Rodi YK, Senhaji O, et al. (2018) Cyclocondensation, Characterization and Antibacterial Activities of Novel 5-Chloro-1HIndole-2,3-Dione Derivatives. J Appl Microbiol Biochem 2(2):1-4.
- Tribak Z, Skalli MK, Senhaji O, Rodi YK (2017) DFT Study and Synthesis of New 1, 2, 3-triazoles Obtained by 1, 3-Dipolar Cycloaddition Derived. 6(7): 1069-1074.
- Düǧdü E, Ünver Y, Ünlüer D, Tanak H, Sancak K, et al. (2013) Synthesis, structural characterization and comparison of experimental and theoretical results by DFT level of molecular structure of 4-(4- methoxyphenethyl)-3,5-dimethyl-4H-1,2,4-triazole. Spectrochim Acta - Part A Mol Biomol Spectrosc 108(2): 329-337.
- Tribak Z, Skalli MK, Senhaji O, Rodi YK (2017) Experimental and Theoretical DFT Study on Synthesis of Novel 5-Chloroisatin Derivatives via 1, 3-Dipolar Cycloaddition Reactions between Allyl-5- chloroindoline-2, 3-dione and 4-Chlorobenzaldoxime. SSRG - IJAC 4(3): 1-6.
- Tribak Z, Kharbach Y, Haoudi A, Skalli MK, Kandri Rodi Y, et al. (2016) Study of new 5-Chloro-Isatin derivatives as efficient organic inhibitors of corrosion in 1M HCl medium: Electrochemical and SEM studies. J Mater Environ Sci 7(6): 2006-2020.
- Tribak Z, Kandri Rodi Y, Haoudi A, Skalli MK, Mazzah AAM, et al. (2017) Cycloaddition 1,3-Dipolaire Des Derives De La 5-Chloro- 1h-Indole-2,3 Dione: Vers De Nouvelles Isoxazolines Et Spirodioxazolines. J Mar Chim Heterocycl 16(1): 59-65.
- Tribak Z, Ghibate R, Skalli MK, Rodi YK, Mrani D, et al. (2017) Synthesis and Characterization of a New Cationic Surfactant Derived from 5-Chloro-1H-indole-2,3-dione In Aqueous Systems. J Eng Res Appl 7(41): 2248-962204.
- Tribak Z, Skalli MK, Senhaji O (2017) Syntheses and Characterizations of Some New N-alkyl, Isoxazole and Dioxazole Derivatives of 5-Chloroisatin 3(8): 882-885.
- Tribak Z, Skalli MK, Senhaji O, Rodi YK. 28071703. 2017;6(8):1145–9.
- Skalli MK, Senhaji O, Rodi YK (2017) Synthesis of 1,2,3-Triazole 5-Chloroisatin Derivatives via Copper-Catalyzed 1,3-Dipolar Cycloaddition Reactions. Int J Adv Eng Manag Sci 3(8): 837-841.
- Tribak Z, Amin O El, Skalli MK, Senhaji O, Rodi YK, et al. (2017) Synthesis, Characterization, and Antibacterial Activity of Some Novel 5-Chloroisatin Derivatives. Int J Eng Res Appl 7(6): 66-70.
- Tribak Z, Kandri Rodi Y, Elmsellem H, Abdel-Rahman I, Haoudi A, et al. (2017) 5-Chloro-1-Octylindoline-2,3-Dione As a New Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solution. J Mater Environ Sci 8(3): 1116-1127.
- Tribak Z, Chda A, Skalli MK, Haoudi A, Rodi YK, et al. (2018) Theoretical approach using DFT and muscle relaxant effects of 5-Chloroisatin Derivatives. Int J Chem Technol 2(2): 105-115.
- Alkorta I, Perez JJ (1996) Molecular polarization potential maps of the nucleic acid bases. Int J Quantum Chem 57(1): 123-135.
- Politzer P, Murray JS (2002) The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor Chem Acc 108(3): 134-142.
- Luque FJ, Orozco M, Bhadane PK, Gadre SR (1993) SCRF calculation of the effect of water on the topology of the molecular electrostatic potential. J Phys Chem 97(37): 9380-9384.
- Kumar R, Keval R, Yadav A, Srivastava CP (2015) Field screening and evaluation of long duration pigeonpea genotypes against the infestation of pod bug. J Pure Appl Microbiol 9(Special Edition 2): 357-364.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...