Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Editorial(ISSN: 2637-4609) 
Determination of Malathion in Water Using Liquid Chromatography & Mass Spectroscopy Volume 1 - Issue 3
Torosyan GH*, Armudjyan EK and Davtyan VA
- National polytechnic university of Armenia, Yerevan
Received: January 25, 2018; Published: February 05, 2018
Corresponding author: Gagik Torosyan, National polytechnic university of Armenia, 37 Sayat-Nova str., apt. 10 Yerevan 375025, Yerevan, Armenia
DOI: 10.32474/AOICS.2018.01.000112
Editorial
One of the most common wastewater pollutants are organo- phosphorus pesticides, which have a complex of properties - high solubility, toxicity, mobility in the environment. In recent years, this method has become an innovative analytical technology for the most part used in medical practice and in pharmaceuticals. With development, the method begins to acquire a practical application in other branches of human life activity [1,2]. In this paper, the results of studies on the detection of Malathion (MLT)-O, O-Dime- thyl-S- (1,2-dicarbethoxyethyl) dithiophosphate in aqueous media. The purpose of this study was to develop liquid chromatography & mass spectroscopy method for the determination of Malathion in water solution. This method will provide experimental results from testing conducted to evaluate sensitivity, selectivity, linearity, precision, accuracy, dilution integrity and various additional stability tests.
Reagents and Equipment
The Experiments were carried out using HPLC Grade Methanol, Acetonitrile and Deionized water supplied by Carl Roth, Germany Formic Acid (98% ACS grade) Carl Roth Germany and Malathion (500 g/L). The research was carried out using Shimadzu's Nexera X2 high-performance liquid-Chromatography equipped with CBM- 20A controller, two Binary LC-30AD Pumps, connected with DGU- 20A5R degasser unit, a SIL-30ACMP auto sampler and column temperature unit CTO-30A (Sciex, Germany). X BridgeC8 1.0 mm x 100 mm dimensions with 3.5|im particle sizes and 136 A pore size column was used (Waters, USA). The LC was coupled with Sciex's Triple Quad™ 4500 Mass spectrometry by the Turbo V™ Ion Source (Sciex, USA). Data processing was performed using the software Analyst 1.6.3 (Sciex, Germany).
Conditions of Mass Spectrometry
The tuning of MS parameters was conducted in positive ionization mode using Sciex's Triple Quad™ 4500 MS for Malathion (MLT). MLT was injected in the MS in 1ng/mL concentration diluted in 1:1 methanol: 0.1% Formic Acid and a flow rate 7|iL/min. and gave predominant singly charged proto nated precursor [M+H] + ions at m/z of 331.1 Da in Q1 full scan spectra. In the product ion mass spectrum of MLT the most abundant and consistent ions were observed at m/z 127.1 Da.
Preparation of Standards Working Solutions
The concentration of the stock solution was 0.5 mg/mL. Two secondary stock solution 5.0 and 1.0|ig/mL were prepared from the stock solution. Serial dilution were made to obtain a final concentration of nine Calibration Curve Standards Working Solutions (CCS WS) as 1.0, 2.0, 5.0, 10.0 20.0 50.0, 100.0, 200.0 and 500.0ng/mL respectively. Four levels of Quality Control Working Solution (QC WS) preparation, separate secondary stocks were prepared and QC WS were diluted to had the concentration 1.0, 3.0, 30.0 and 150.0for Lower Limit of Quantization QC (LLOQ QC), Low Quality Control (LQC), Medium Quality Control (MQC) and High-Quality Control (HQC) respectively. Internal Standard (IS) was chosen to be Solifenacin Succinate, one of the laboratory standards, and the Working Solution of the IS was prepared to have the concentration of 20ng/mL.
Sample Preparation
Sample Preparation was a one-step dilutes and shoot. Into 900|iL Deionized Water, 50|iL of IS and 50|iL of each CCS/QC was pipette into each sample. To be diluted twenty times than the working Solutions concentration. Those samples were prepared in 1.8mL glass vials and after mixing they had placed in the auto sampler of the LC. The final concentrations were as the following for Calibration Curve Standards (CCS) 0.05, 0.1, 0.25, 0.50, 1.00, 2.50, 5.00 and 10.00ng/mL, and for Quality Control Samples (QC) 0.05, 0.15, 1.50 and 7.5ng/mL respectively.
Method Performance Check Results
For checking the performance of the method, precision and accuracy tests were conducted. Precision of an assay is defined as the closeness of the results when replicate assays of a test sample are carried out. The precision is expressed as %CV. The accuracy of a method is defined as the proximity of the determined values to actual concentrations of the samples. The accuracy is expressed as % Accuracy. Both the inter-run and intra-run precision and accuracy were assessed by replicate analysis of samples containing known amounts of the Analyte (QCs) . The results for inter-run and intra-run precision and accuracy tests of four replicate QC samples at LLOQ QC, LQC, MQC and HQC concentration levels are listed in (Tables 1 & 2). Assay sensitivity was assessed by precision (%CV), accuracy (%Nominal) and signal to noise (S/N) ratio at CCS1 concentration of all Calibration Curves. The results are listed in (Table 3).
Table 1: Accuracy Results.
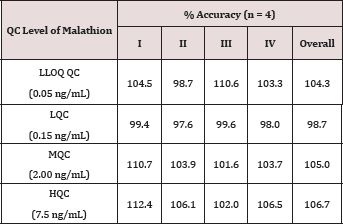
Table 2: Precision Results.
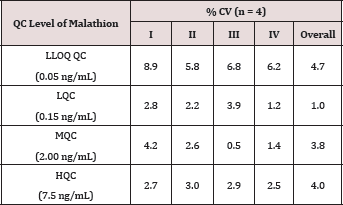
Table 3: Sensitivity Results.

Conclusion
The developed method was proved to be fast and efficient, more over this method showed to be simple, rugged, sensitive and selective for the quantitative determination of Malathion in aqueous solution using Liquid Chromatography tandem Mass Spectrometry. Thus, this method can be used for widespread environmental analysis for determination of pesticides in water
Acknowledgement
Darmantest Laboratories are acknowledged for the support and the help they provided to develop and process this method.
References

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...