Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4544
Research Article(ISSN: 2637-4544) 
Role IVF in Preeclampsia Pathogenesis: impact of extracellular vesicles Volume 5 - Issue 5
Murat Basar 1,2* and Yagmur Ergun2
- 1Department of Obstetrics, Gynecology, and Reproductive Sciences, Yale School of Medicine, New Haven, CT, USA
- 2Yale Fertility Center, 200 West Campus Drive, Orange, USA
Received:June 14, 2023;Published:June 22, 2023
Corresponding author:Murat Basar, PhD, Department of Obstetrics, Gynecology, and Reproductive Sciences, Yale Fertility Center, 200 West Campus Drive Orange, CT 06477
DOI: 10.32474/IGWHC.2023.05.000221
Abstract
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
Preeclampsia (PE) is a complex and life-threatening pregnancy complication affecting both mother and fetus. Recent studies have shown a possible link between PE and in Vitro Fertilization (IVF) treatments, suggesting that Extracellular Vesicles (EVs) play a crucial role in the pathophysiology of PE [1,2]. This review aims to provide a comprehensive understanding of the involvement of EVs in the development of PE, as well as their potential role in IVF-related PE. We also explore the potential use of EVs as diagnostic biomarkers and therapeutic targets for PE in the context of Assisted Reproductive Technologies (ART).
Keywords: Preeclampsia; Extracellular Vesicles; Vitro Fertilization; Reproductive Technologies; Female Reproductive System
Introduction
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
Preeclampsia is a multifactorial pregnancy complication affecting 3-8% of pregnancies worldwide [3], characterized by the onset of hypertension and proteinuria after 20 weeks of gestation. This disorder severely affects maternal and fetal health, leading to preterm birth, Intrauterine Growth Restriction (IUGR), and even maternal and fetal death [4]. Despite advances in obstetric care, the etiology and pathophysiology of PE remain elusive, making early diagnosis and effective treatments challenging. In recent years, Assisted Reproductive Technologies (ART), Including in Vitro Fertilization (IVF), have increased dramatically. Several studies have reported a higher incidence of PE among women who underwent IVF treatments [5]. This association suggests a potential role of Extracellular Vesicles (EVs) in developing PE, as they have been identified as crucial mediators of cell-tocell communication in various physiological and pathological processes, including pregnancy [6]. Recent advances in Assisted Reproductive Technologies (ART), such as in Vitro Fertilization (IVF), have increased success rates for infertile couples. However, these technologies have also been associated with a higher risk of pregnancy complications, including Preeclampsia (PE) [7]. The involvement of Extracellular Vesicles (EVs) in the Pathogenesis of PE, particularly in the context of IVF, warrants further investigation to better understand this relationship and identify potential diagnostic and therapeutic strategies for PE. This review provides a comprehensive overview of the current knowledge on the role of EVs in PE, with a particular focus on their potential involvement in IVF-related PE, and discusses future perspectives and challenges in the field. This review will focus on the role of EVs in the development of PE, particularly in the context of IVF, and discuss the potential use of EVs as diagnostic biomarkers and therapeutic targets for PE.
Preeclampsia: An Overview
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
Pathophysiology and Risk Factors
The Pathophysiology of PE is complex and multifactorial, involving genetic, immunological, and environmental factors [8]. The most widely accepted theory involves abnormal placentation, leading to placental ischemia and the subsequent release of soluble factors into the maternal circulation, promoting endothelial dysfunction, inflammation, and, ultimately, the clinical manifestations of PE [9]. Key risk factors for PE include nulliparity, advanced maternal age, obesity, pre-existing medical conditions (e.g., hypertension, diabetes, renal disease), and history of PE in previous pregnancies [10]. Additionally, IVF treatments have emerged as a potential risk factor for developing PE [11].
Clinical Presentation and Diagnosis
PE is a heterogeneous disorder with a wide range of clinical presentations, from mild to severe [12]. The main clinical features include new-onset hypertension and proteinuria after 20 weeks of gestation [13]. Other symptoms include edema, headache, visual disturbances, and epigastric pain [14]. The diagnosis of PE is primarily based on clinical and laboratory findings [15]. However, due to the lack of specific biomarkers, early diagnosis and differentiation from other hypertensive disorders of pregnancy remain challenging [16].
Management and Treatment
The management of PE primarily focuses on controlling maternal blood pressure, preventing seizures (eclampsia), and monitoring fetal well-being [17]. Antihypertensive medications are commonly used to manage blood pressure, while magnesium sulfate is administered to prevent seizures [18]. Fetal surveillance, including ultrasound and non-stress tests, is essential for monitoring fetal well-being and guiding delivery timing [19]. In severe cases, early delivery may be necessary to protect the health of the mother and the fetus [20]. However, this can lead to complications associated with preterm birth, such as respiratory distress syndrome and neonatal intensive care unit admission [2].
Prevention Strategies
Current preventive strategies for PE include the administration of low-dose aspirin for women at high risk of developing the disorder [21]. Aspirin has been shown to reduce the risk of PE by 10- 20% when initiated before 16 weeks of gestation [22]. Identifying specific risk factors, such as those related to IVF, could help tailor prevention strategies for individual patients and improve outcomes.
Extracellular Vesicles: Biogenesis, Classification, and Functions
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
Biogenesis and Classification
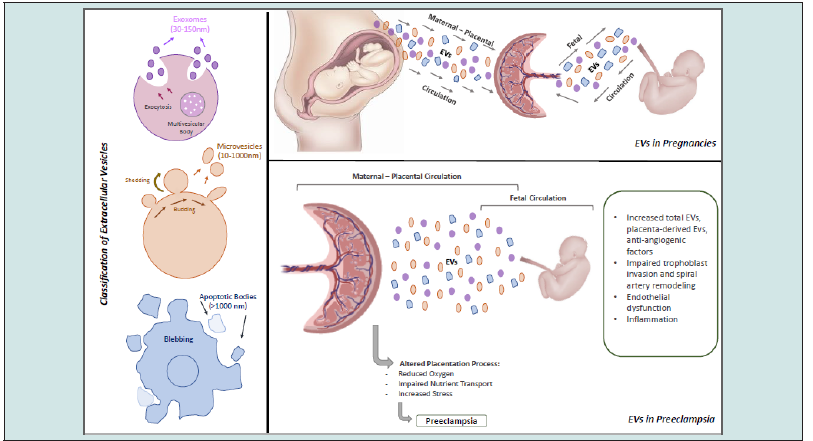
Extracellular vesicles are lipid bilayer-enclosed particles released by cells into the extracellular environment [23]. They are formed by the inward budding of the endosomal membrane (exosomes) or the outward budding of the plasma membrane (microvesicles) [24]. EVs can be classified into three main categories based on their size, biogenesis, and molecular markers: exosomes (30-150 nm), microvesicles (100-1000 nm), and apoptotic bodies (500-2000 nm) [25].
Functions of Extracellular Vesicles
EVs have diverse functions in intercellular communication, immune modulation, and tissue homeostasis [26]. They can carry various bioactive molecules, including proteins, lipids, and nucleic acids, which can be transferred to recipient cells, influencing their function [27]. In the context of pregnancy, EVs have been implicated in processes such as placental development, maternal-fetal immune tolerance, and the maintenance of pregnancy [28].
Extracellular Vesicle Cargo
The cargo of EVs consists of various bioactive molecules, including proteins, lipids, and nucleic acids, which can be transferred to recipient cells and modulate their function [29]. The cargo composition is determined by the cell of origin, the cellular state, and the physiological or pathological context [30]. In pregnancy, EV cargo can include placental proteins, hormones, and regulatory RNAs, which may contribute to pregnancy-related processes and complications, such as PE [31].
Isolation and Characterization of Extracellular Vesicles
The isolation and characterization of EVs from biological fluids, such as blood and urine, have been challenging due to their small size and heterogeneous nature [32]. Several techniques have been developed for EV isolation, including ultracentrifugation, size-exclusion chromatography, and immunoaffinity capture [33]. Each method has advantages and limitations, and the choice of technique depends on the specific research question and the intended downstream analysis [34]. Following isolation, EVs can be characterized by their size, morphology, and molecular markers using nanoparticle tracking analysis, transmission electron microscopy, and flow cytometry [6].
Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
In the Vagina
These vesicles, known as vaginosomes, have been demonstrated to affect sperm capacitation and acrosome response in mice, similar to EVs reported in other female biofluids [35]. There is some evidence that extracellular RNAs present in the vagina, notably miR-186-5p, can guard against HIV-1 infection [36].
In the Uterus
Uterosomes are extracellular vesicles that can be found in the uterus’ luminal fluid [37]. Uterosome-related proteins, which are secreted by endometrial epithelial cells, appear to be engaged in crucial embryo-implantation pathways, indicating that these vesicles are vital in early pregnancy [38]. In addition to being secreted by endometrial cells, uterosomes have been demonstrated to be taken up by endometrial epithelial cells and profoundly change their transcriptome[39].
In the Ovaries
The fluid surrounding an expanding oocyte, the cell in an ovary that can develop into an ovum, is known as Follicular Fluid (FF). Blood plasma components that penetrate the “blood-follicle barrier” and the secretory activity of granulosa and thecal cells contribute to producing follicular fluid [40]. The Cumulus-Oocyte Complex (COC) grows just before ovulation, enabling the egg to complete meiotic maturation. This is likely the result of communication between granulosa cells and the COC. Many studies show that the EVs released from the FF can mediate several mechanisms, such as the transforming growth factor-β (TGF-β) pathway [41]. COC expansion mechanism can be mediated by the miRNAs released within these EVs; human-derived EVs contain miRNAs that target genes involved in follicular maturation inhibition and meiosis resume [42, 43]. On the other hand, other studies suggest that differences in the impact of EVs may be allocated to variations in their molecular cargo at different periods of the menstrual cycle [44, 45]. EVs have also been demonstrated to be taken up by and modify the transcriptome of epithelial cells that line the fallopian tubes, resulting in the expression of genes that increase the chance of fertilization and embryo development [46].
Role of Extracellular Vesicles in Normal Pregnancies
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
Fertilization and EVs
The female reproductive system undergoes sperm capacitation, also called sperm activation, which starts the signaling pathways required for the sperm to penetrate the several layers of the female egg. Sperm cells receive plasma membrane Ca2+-ATPase 4a (PMCA4a) and PMCA1 from oviductosomes, uterosomes, and vagisomes during the sperm capacitation process [47,48]. EVs released from the oviduct and uterus have also been demonstrated to transmit tyrosine phosphorylated proteins to sperm, which may alter capacitation [37,47]. In Vitro Fertilization (IVF) relies on adding sperm to a collected egg; however, the oocyte must be completely developed before fertilization. It has been demonstrated that incubating retrieved oocytes with follicular fluid-derived EVs and/or oviductosomes enhances oocyte maturation and embryonic development [49,50].
Implantation, Maternal-Fetal Crosstalk and EVs
Following fertilization, the conceptus trophectoderm releases EVs into the uterine fluid, and these vesicles are thought to help facilitate communication between the endometrial lining and the fertilized egg before implantation [51,52]. EVs are a crucial mediator of the bidirectional connection between the endometrial and trophoblast cells, allowing for the transfer of vital cargo to promote embryo implantation, such as angiogenic and proliferative factors [53]. Recent data reveals that lower-grade embryos produce more EVs than higher-quality embryos, and these EVs are often smaller in diameter, suggesting that the amount and size of EVs released from IVF embryos may be a sign of embryo quality [54-56]. Co-culturing IVF embryos generates a microenvironment that utilizes paracrine communication, resulting in better embryonic development than independently cultured embryos [57]. These EVs, which enhance the developmental competence of co-cultured embryos and include the pluripotency genes Nanog, Klf4, Oct4, Sox2, and c-Myc, may be partially responsible for this phenomenon [58]. Early gestation is a critical time for cellular communication at the maternal-fetal interface, which controls pregnancy outcomes. The endometrium and growing conceptus may rapidly exchange biomolecules when placentation is successful. Besides, previous data indicate that EVs are essential in modifying maternal immunity and enabling immunological tolerance to fetal antigens, which lowers the chance of rejection/abortion [59]. When EVs are floating freely in the mother’s bloodstream, the proteins carried by trophoblastic EVs may perform a dual function by inhibiting complement activation and controlling the activity of maternal T cells, which could otherwise result in unfavorable immune reactions to paternally derived antigens expressed by the placenta [60
Placentation And Evs
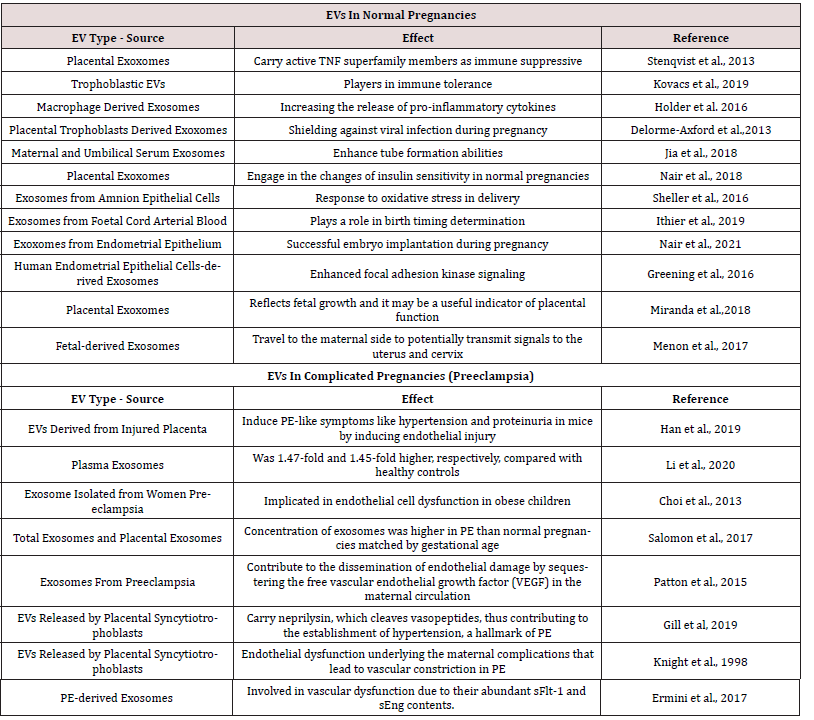
In humans, between weeks 10 and 12, the placenta, which sustains the fetus for several months and serves as the primary transporter of oxygen and nutrients for the developing fetus, connects to the mother’s uterus by remodeling the spiral arteries along the uterine wall. This remodeling is facilitated by extravillous cytotrophoblasts [61]. Within this context, extravillous trophoblasts have been demonstrated to migrate into the decidua of the uterus using EVs produced from cytotrophoblasts [62]. It has been shown that EVs generated from the placenta express the immunomodulatory proteins GLA-G5, B7-H1, and B7-H3, which can influence T cell responses and may be related to maternal-fetal tolerance [63]. Recent research reveals that placental-derived EVs interact with maternal lung and liver immune cells through surface integrins [64]. Bioengineered EVs were injected into pregnant mice, and the results revealed EV trafficking to fetal cells, indicating that maternal EVs may cross the placenta and affect the baby [65] (Table 1).
Role of Extracellular Vesicles in Preeclampsia
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
Altered Extracellular Vesicle Profile in Preeclampsia
Several studies have reported an altered EV profile in the maternal circulation of women with PE compared to normotensive pregnant women [31]. Specifically, women with PE have increased total EVs, placenta-derived EVs, and EVs containing proinflammatory and anti-angiogenic factors [66] (Figure 1).
Placental Extracellular Vesicles in Preeclampsia
Placenta-derived EVs play a crucial role in the pathophysiology of PE [67]. They carry various bioactive molecules that can contribute to endothelial dysfunction and systemic inflammation [2]. In PE, the placenta releases more EVs, containing factors such as soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng), which are known to induce maternal vascular dysfunction [18].
Potential Mechanisms Linking Extracellular Vesicles to Preeclampsia Pathogenesis
EVs have been implicated in several pathways related to the
pathogenesis of PE, including:
a. Impaired trophoblast invasion and spiral artery
remodeling: Placental EVs can modulate the invasion of Extravillous
Trophoblasts (EVTs) into the maternal decidua and spiral arteries
[68]. In PE, EVs may carry factors that inhibit trophoblast invasion
and impair spiral artery remodeling, leading to placental ischemia
[69].
b. Endothelial dysfunction: EVs containing anti-angiogenic
factors, such as sFlt-1 and sEng, can disrupt the balance of
angiogenic and anti-angiogenic factors, contributing to endothelial
dysfunction in PE [30].
c. Inflammation: Pro-inflammatory factors carried
by EVs can promote systemic inflammation, which further
exacerbates endothelial dysfunction and contributes to the clinical
manifestations of PE [22].
Extracellular Vesicles in the Maternal-Fetal Interface
The maternal-fetal interface is critical for maintaining pregnancy and ensuring proper fetal development [26]. EVs have been implicated in regulating maternal-fetal communication and immune tolerance at this interface [70]. In PE, the altered EV profile at the maternal-fetal interface may contribute to the breakdown of immune tolerance and the subsequent development of the disorder [6].
Long-term Consequences of Preeclampsia and Extracellular Vesicles
Preeclampsia has been associated with long-term health consequences for both the mother and the offspring [27]. Women who have experienced PE are at an increased risk of developing cardiovascular diseases later in life, such as hypertension, ischemic heart disease, and stroke [71]. Offspring born to mothers with PE are at a higher risk of developing metabolic and cardiovascular disorders, including hypertension, diabetes, and obesity [72]. The role of EVs in mediating these long-term consequences is not yet fully understood, but the altered EV profile during pregnancy may have lasting effects on maternal and offspring health [73].
Extracellular Vesicles in IVF and Preeclampsia
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
Increased Incidence of Preeclampsia in IVF Pregnancies
Several studies have reported an increased risk of PE among women who conceived through IVF [74]. The reasons for this increased risk are partially clear. However, it has been suggested that IVF-related factors may contribute to the development of PE [17].
Potential Role of Extracellular Vesicles in IVF-Related Preeclampsia
The altered EV profile observed in PE may also be present in IVF pregnancies, suggesting a potential role of EVs in the development of IVF-related PE [75]. Further research is needed to determine this association’s specific factors and mechanisms.
Extracellular Vesicles in Ovarian Stimulation and Embryo Culture
Ovarian stimulation and embryo culture are essential components of IVF treatments that may influence the EV profile in maternal circulation [76]. Ovarian stimulation with exogenous hormones can alter the local EV profile in the follicular fluid, which may affect oocyte quality and embryo development [77]. Additionally, embryo culture conditions, including specific growth factors and cytokines, can influence the EVs released by the developing embryo and the surrounding environment [78]. The potential impact of these IVF-related factors on the EV profile and the subsequent risk of PE warrants further investigation.
Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
Extracellular Vesicles as Diagnostic Biomarkers
Given the altered EV profile in PE, EVs have been proposed as potential diagnostic biomarkers for early disorder detection [79]. Studies have shown that specific placenta-derived EVs and EV-associated factors such as sFl t-1 and sEng can be detected in maternal circulation before clinical symptoms, suggesting their potential use in early diagnosis and risk stratification of PE [80]. However, further research is needed to validate these findings and establish standardized EV isolation and analysis methods in a clinical setting.
Extracellular Vesicles as Therapeutic Targets
The involvement of EVs in the pathophysiology of PE also
presents opportunities for therapeutic intervention. Potential
strategies include:
a. Modulation of EV release: Targeting the molecular
mechanisms responsible for the release of placenta-derived
EVs could help to reduce the number of harmful EVs in maternal
circulation [80].
b. Inhibition of EV uptake: Blocking the uptake of placentaderived
EVs by maternal cells may prevent the deleterious effects of
their bioactive cargo on maternal vascular function [31].
c. Neutralization of EV cargo: Therapeutic agents, such as
antibodies or small molecules, could be developed to neutralize the
pro-inflammatory and anti-angiogenic factors carried by placentaderived
EVs in PE [81].
d. Replacement of defective EVs: Administration of
exogenous EVs with a “healthy” cargo may help to restore the
balance of angiogenic and anti-angiogenic factors and promote
proper placental function [82].
Challenges and Limitations of Extracellular Vesicle- Based Diagnostics and Therapeutics
Despite the promising potential of EVs as diagnostic biomarkers and therapeutic targets for PE, several challenges and limitations must be addressed. One major challenge is EV population heterogeneity, making it difficult to identify specific EV subpopulations and their molecular signatures associated with PE [83]. Additionally, the need for standardized EV isolation, characterization, and quantification methods presents a significant obstacle to the reproducibility and comparability of findings across different studies [84]. Furthermore, the safety and efficacy of EVbased therapeutics must be carefully evaluated in preclinical and clinical studies before their implementation in clinical practice [85].
Future Perspectives and Challenges
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
The study of extracellular vesicles in the context of preeclampsia and IVF has opened new avenues for understanding the pathophysiology of this complex disorder and the potential links between assisted reproductive technologies and adverse pregnancy outcomes. However, several challenges remain to overcome before EVs can be fully harnessed for diagnostic and therapeutic purposes. Firstly, standardization of EV isolation, characterization, and quantification methods is crucial to ensure the reproducibility and comparability of findings across different studies [86]. Secondly, identifying specific EV subpopulations and their molecular signatures in preeclampsia will be essential for developing targeted diagnostic and therapeutic strategies [87]. Translating EV-based interventions into clinical practice will require extensive preclinical and clinical evaluation to ensure their safety and efficacy [86]. Advances in EV research will be crucial for overcoming the current challenges associated with the study of EVs in PE and IVF [88]. Moreover, interdisciplinary collaborations between reproductive medicine specialists, EV researchers, and bioengineers will be essential for translating emerging insights into clinically relevant diagnostic and therapeutic applications [89]. Finally, further research is needed to determine the long-term consequences of altered EV profiles in PE and IVF on maternal and offspring health and the potential benefits of early intervention strategies based on EV biology [90].
Conclusion
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
Extracellular vesicles have emerged as essential players in the pathophysiology of preeclampsia, particularly in IVF. The altered EV profile observed in women with PE and their roles in placental development, endothelial dysfunction, and inflammation suggests that EVs may serve as promising diagnostic biomarkers and therapeutic targets for this complex disorder. Further research is needed to elucidate the specific mechanisms linking EVs to preeclampsia and to overcome the challenges associated with their clinical application [7]. Extracellular vesicles have emerged as critical players in the pathophysiology of preeclampsia, particularly in IVF. The altered EV profile observed in women with PE and their roles in placental development, endothelial dysfunction, and inflammation suggests that EVs may serve as promising diagnostic biomarkers and therapeutic targets for this complex disorder. Further research is needed to elucidate the specific mechanisms linking EVs to preeclampsia, to identify the factors and pathways involved in IVF-related PE, and to overcome the challenges associated with the clinical application of EVs in the diagnosis and treatment of PE [91].
Results
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
Socio-demographic variables of respondents
A total number of 228 women of reproductive age attending
the gynecological clinic in the University College Hospital, Ibadan
participated in the study. The average age of respondents was
32.7, with a standard deviation of 6.47. The highest percentage
(58.1%) of the respondents was within 30-39 years, and 89.4% of
the respondents were married, and 92.5% were employed. Also,
93.4% had a post-secondary academic qualification, and 4.8% had
secondary school qualification. Others, as shown in (Table 1).
Respondents on knowledge and uptake of CCS
Most of the respondents (82.9%) ever heard of cervical cancer,
96.5% had no history of cervical cancer, 46.5% knew any CCS
procedure with 45.0% reporting Pap smear test. Also, 31.6% of
respondents reported that a person should start CCS when such
a person is sexually active, 29.0% said that one should start CCS
when that individual is 18 years and above.
Also, only 22.4% had ever gone for CCS, 8.6% reported
preventive measure as the reason they went for CCS. However,
10.4% reported that they had gone for CCS only once in their
lifetime with 10.0% reporting they went for CCS when they were
within the age bracket of 20-29 years. (Table 2) highlighted others.
Furthermore, regression analysis shows that the level of knowledge
had no significant influence (R2=0.10, F (1,227) =1.987, P >.05) on
the uptake of CCS.
Respondents’ responses to risk factors (perceived susceptibility)
Table 3 shows the risk factors that are associated with cervical cancer, 37.7% of the respondents reported sexually transmitted diseases, poor hygiene (25.9%), positive family history (31.6%), 30.7% had several sexual partners, early age of first sexual intercourse (18.9%), cigarette smoking (18.4%), contraceptives (17.5%) and HIV/AIDS (13.6%). Regression analysis shows that perceived susceptibility had significant influence (R2=0.92, F (1,227) =16.022, P <.001) on the uptake of CCS.
Respondents’ responses to perceived benefits
(Table 4) shows that (62.7%) of the respondents strongly agreed that it is essential to have CCS to now if one is healthy, 46.1% strongly agreed that CCS could find changes in the cervix before becoming cancerous, and 47.8% strongly agreed that changes found from CCS are easily curable. Regression analysis shows that perceived benefits had no significant influence (R2=0.007, F (1,227) =1.396, P >.05) on the uptake of CCS.
Table 4: Showing participants’ responses to perceived benefits.

SA-Strongly Agreed, A-Agreed, D-Disagree, SD-Strongly Disagreed, IDK- I do not know; F/% - Frequency and Percentage
Respondents’ responses to perceived barriers
About (32.9%) reported lack of information about CCS procedures, 14.9% reported not knowing where to go for CCS, tests are costly (11.4%), cervix is part of the sex organ and it is private (11.4%), lack of female screeners (10.1%), attitude of health workers (10.1%), and lack of convenient screening time (10.1%). (Table 5) highlighted others. The regression analysis shows that perceived barriers had no significant influence (R2=0.00, F (1,227) =0.34, P >.05) on the uptake of CCS.
Table 5: Showing participants’ responses to perceived barriers.

SA-Strongly Agreed, A-Agreed, D-Disagree, SD-Strongly Disagreed; F/% - Frequency and Percentage
Respondents’ responses to cues to action on CCS
Almost half (48.7%) reported take care of my health, (29.8%) after hearing something about CC, (20.2%) because a nurse or mid wife told me, (17.1%) listened to radio or read newspaper, (15.8%) because a doctor told me, and (14.0%) saw it on social media platforms. Table 6 reported others. The regression analysis shows that cues to action had no significant influence (R2=0.007, F (1,227) =1.358, P >.05) on the uptake of CCS.
Table 6: Showing participants’ responses to Cues to Action on CCS.

SA-Strongly Agreed, A-Agreed, D-Disagree, SD-Strongly Disagreed; F/% - Frequency and Percentage
Discussion
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
The participants’ level of knowledge reported in this study is contrary to previous findings. [15] Despite having over nine out of ten participants with post-secondary educational qualification, one would expect a significant level of knowledge about cervical cancer among them. However, the findings of WHO in the year 2012 13 among six sub-Saharan nations also established a low level of awareness of cervical cancer, which led to low uptake of CCS uptake [7]. Also, the studies have substantiated this finding and emphasized that the level of CCS uptake among females in Nigeria is very discouraging [16, 17]. The fact that an individual attained a higher level of education does not necessarily culminate in having excellent knowledge about health issues, let alone taking appropriate preventive steps.
The perceived susceptibility reported by the participants has a significant effect on the uptake of CCS, which shows that the perception that one is at risk of cervical cancer could motivate such individual to undertake CCS. On the other hand, individuals might not undertake CCS if they perceive that they are not susceptible to cervical cancer. As a result, significant perceived susceptibility would positively influence the essence of taking preventive measures towards cervical cancer. The findings of previous studies supported this finding. [8, 18] Among perceived barriers on uptake of CCS reported by the participants of this study is the lack of female screeners, which makes it uncomfortable and painful for women to allow men to examine them. Previous studies established that women prefer female physician to perform CCS [19, 20]. Also, the lack of information plays a significant role in the low uptake of CCS. Since they know next to nothing about the availability of CCS, one would not expect them to go for screening. To corroborate this finding, Ndikom and Ofi [14] also found a lack of information as a perceived barrier among the participants. The studies conducted by other researchers established a lack of information as the main predicting barriers of uptake of CCS among African women [16, 21, 22].
Furthermore, perceived benefits reported by the participants had no significant effect on the uptake of CCS. Their perceived benefits did not culminate to practice, which shows that the participants have low perceived benefits of CCS. In other words, they place insignificant value on what they stand to gain from CCS. Other study also reported similar findings among Saudi Arabian women. [20]
The final finding shows that cues to action reported by the participants did not result in uptake of CCS. Though some participants strongly agreed that taking care of their health is a cue to action; 47.8% (n=109) agreed that hearing something about cervical cancer is a cue to action, and 17.1% (n=39) reported getting information from the media while 14.0% (n=32) reported social media as their source. Still, the cues to action reported did not translate into practice. This finding is contrary to the previous work, [23] where significant others and healthcare professionals influenced the uptake of CCS among the respondents.
Conclusion
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
Findings from the present study revealed a low level of knowledge about cervical cancer and the level of knowledge had no significant influence on the uptake of CCS. However, the participants’ perceived susceptibility significantly modified CCS uptake. Furthermore, perceived barriers had no significant effect on the uptake of CCS. Similarly, perceived benefits had no significant effect on the uptake of CCS.
Recommendation
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
The study recommends community-integrated cervical cancer screening program, which will be available, accessible and affordable for all women regardless of their socio-economic status. Such should be planned with the intention of creating more awareness and increase the present level of knowledge about cervical cancer and its consequences so that women would better comprehend how susceptible they are to the disease and as such take appropriate steps.
References
- Abstract
- Introduction
- Preeclampsia: An Overview
- Extracellular Vesicles: Biogenesis, Classification, and Functions
- Extracellular Vesicles in Female Reproductive System: Origin of Releases
- Role of Extracellular Vesicles in Normal Pregnancies
- Role of Extracellular Vesicles in Preeclampsia
- Extracellular Vesicles in IVF and Preeclampsia
- Diagnostic and Therapeutic Potential of Extracellular Vesicles in Preeclampsia
- Future Perspectives and Challenges
- Conclusion
- References
- Redman CW, Sargent IL (2005) Latest advances in understanding preeclampsia. Science 308(5728): 1592-1594.
- Suchismita Sarker, Katherin Scholz-Romero, Alejandra Perez, Sebastian E Illanes, Murray D Mitchell, et al. (2014) Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med 12: 204.
- Edgardo Abalos, Cristina Cuesta, Ana L Grosso, Doris Chou, Lale Say (2013) Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol 170(1): 1-7.
- Eric A P Steegers, Peter von Dadelszen, Johannes J Duvekot, Robert Pijnenborg (2010) Pre-eclampsia. Lancet 376(9741): 631-644.
- Liv Bente Romundstad, Romundstad Pa R, Arne Sunde, Vidar von During, Rolv Skjaerven, et al. (2008) Effects of technology or maternal factors on perinatal outcome after assisted fertilisation: a population-based cohort study. Lancet 372(9640): 737-743.
- Maria Yáñez-Mó, Pia R-M Siljander, Zoraida Andreu, Apolonija Bedina Zavec, Francesc E Borràs, et al. (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 14(4): 27066.
- Christianne AR Lok, Jiska Jebbink, Rienk Nieuwland, Marijke M Faas, Kees Boer, et al. (2009) Leukocyte activation and circulating leukocyte-derived microparticles in preeclampsia. Am J Reprod Immunol 61(5): 346-359.
- Ivo Brosens, Robert Pijnenborg, Lisbeth Vercruysse, Roberto Romero (2011) The "Great Obstetrical Syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol 204(3): 193-201.
- Burton GJ, E Jauniaux (2004) Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig 11(6): 342-352.
- Duckitt K, Harrington D (2005) Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ 330(7491): 565.
- Shilpi Pandey, Ashalatha Shetty, Mark Hamilton, Siladitya Bhattacharya, Abha Maheshwari (2012) Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update 18(5): 485-503.
- Lindheimer MD, Taler SJ, Cunningham FG (2008) Hypertension in pregnancy. J Am Soc Hypertens 2(6): 484-494.
- (2013) Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol 122(5): 1122-1131.
- Laura A Magee, Anouk Pels, Michael Helewa, Evelyne Rey, Peter von Dadelszen (2014) Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can 36(7): 416-441.
- (2019) Hypertension in pregnancy: diagnosis and management. London.
- Rana S (2019) Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ Res 124(7): 1094-1112.
- Tina Cronqvist, Dionne Tannetta, Matthias Mörgelin, Mattias Belting, Ian Sargent, et al. (2017) Syncytiotrophoblast derived extracellular vesicles transfer functional placental miRNAs to primary human endothelial cells. Sci Rep 7(1): 4558.
- Murray D Mitchell, Hassendrini N Peiris, Miharu Kobayashi, Yong Q Koh, Gregory Duncombe, et al. (2015) Placental exosomes in normal and complicated pregnancy. Am J Obstet Gynecol 213(4 Suppl): S173-S181.
- Francesca Balzano, Marta Deiana, Silvia Dei Giudici, Annalisa Oggiano, Angela Baralla, et al. (2015) miRNA Stability in Frozen Plasma Samples. Molecules 20(10): 19030-19040.
- Rebecca A Dragovic, Jennifer H Southcombe, Dionne S Tannetta, Christopher WG Redman, Ian L Sargent (2013) Multicolor flow cytometry and nanoparticle tracking analysis of extracellular vesicles in the plasma of normal pregnant and pre-eclamptic women. Biol Reprod 89(6): 151.
- Redman CW, Sargent IL (2010) Immunology of pre-eclampsia. Am J Reprod Immunol 63(6): 534-543.
- Carlos Salomon, Maria Jose Torres, Miharu Kobayashi, Katherin Scholz Romero, Luis Sobrevia, et al. (2014) A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One 9(6): e98667.
- Clotilde Théry, Kenneth W Witwer, Elena Aikawa, Maria Jose Alcaraz, Johnathon D Anderson, et al. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1): 1535750.
- Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200(4): 373-383.
- van Niel G, Angelo GD, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19(4): 213-228.
- Tkach M, Thery C (2016) Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 164(6): 1226-1232.
- Hadi Valadi, Karin Ekström, Apostolos Bossios, Margareta Sjöstrand, James J Lee (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6): 654-659.
- Carlos Salomon, Sarah Yee, Katherin Scholz Romero, Miharu Kobayashi, Kanchan Vaswani, et al. (2014) Extravillous trophoblast cells-derived exosomes promote vascular smooth muscle cell migration. Front Pharmacol 5: 175.
- R A Dragovic, G P Collett, P Hole, Ferguson DJP, C W Redman, et al. (2015) Isolation of syncytiotrophoblast microvesicles and exosomes and their characterisation by multicolour flow cytometry and fluorescence Nanoparticle Tracking Analysis. Methods 87: 64-74.
- Y Ouyang, Mouillet JF, Coyne CB, Sadovsky Y (2014) Review: placenta-specific microRNAs in exosomes - good things come in nano-packages. Placenta 35 Suppl: S69-S73.
- Dionne Tannetta, Ieva Masliukaite, Manu Vatish, Christopher Redman, Ian Sargent (2017) Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia. J Reprod Immunol 119: 98-106.
- Coleman BM, Hill AF (2015) Extracellular vesicles--Their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Semin Cell Dev Biol 40: 89-96.
- Yanhua Tian, Suping Li, Jian Song, Tianjiao Ji, Motao Zhu, et al. (2014) A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35(7): 2383-2390.
- Thomas Lener, Mario Gimona, Ludwig Aigner, Verena Börger, Edit Buzas, et al. (2015) Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 4: 30087.
- Zeinab Fereshteh, Pradeepthi Bathala, Deni S Galileo, Patricia A Martin DeLeon (2019) Detection of extracellular vesicles in the mouse vaginal fluid: Their delivery of sperm proteins that stimulate capacitation and modulate fertility. J Cell Physiol 234(8): 12745-12756.
- Zezhou Zhao, Dillon C Muth, Kathleen Mulka, Zhaohao Liao, Bonita H Powell, et al. (2020) (miRNA profiling of primate cervicovaginal lavage and extracellular vesicles reveals miR-186-5p as a potential antiretroviral factor in macrophages. FEBS Open Bi 10(10): 2021-2039.
- A Franchi, Cubilla M, Guidobaldi HA, Bravo AA, Giojalas LC (2016) Uterosome-like vesicles prompt human sperm fertilizing capability. Mol Hum Reprod 22(12): 833-841.
- Gregory Burns, Kelsey Brooks, Mark Wildung, Raphatphorn Navakanitworakul, Lane K Christenson, et al. (2014) Extracellular vesicles in luminal fluid of the ovine uterus. PLoS One 9(3): e90913.
- Renwu Hua, Qiaorui Liu, Weisi Lian, Dengying Gao, Cheng Huang , et al. (2022) Transcriptome regulation of extracellular vesicles derived from porcine uterine flushing fluids during peri-implantation on endometrial epithelial cells and embryonic trophoblast cells. Gene 822: 146337.
- Gosden RG, Hunter RH, Telfer E, Torrance C, Brown N (1988) Physiological factors underlying the formation of ovarian follicular fluid. Reproduction 82(2): 813-825.
- Drummond AE (2005) TGFbeta signalling in the development of ovarian function. Cell Tissue Res 322(1):107-115.
- Manuela Santonocito, Maria elena Vento, Maria R Guglielmino, Rosalia Battaglia, Jessica Wahlgren, et al. (2014) Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril 102(6): 1751-1761.
- Juliano C da Silveira, Rao Veeramachaneni DN, Quinton A Winger, Elaine M Carnevale, Gerrit J Bouma (2012) Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod 86(3): 71.
- Elisa M Pioltine, Mariana F Machado, Juliano C da Silveira, Patrícia K Fontes, Ramon C Botigelli, et al. (2020) Can extracellular vesicles from bovine ovarian follicular fluid modulate the in-vitro oocyte meiosis progression similarly to the CNP-NPR2 system? Theriogenology 157: 210-217.
- Ana Clara Faquineli Cavalcante Mendes de Ávila, Alessandra Bridi, Gabriella Mamede Andrade, Maite Del Collado, Juliano Rodrigues Sangalli, et al. (2020) Estrous cycle impacts microRNA content in extracellular vesicles that modulate bovine cumulus cell transcripts during in vitro maturation†. Biol Reprod 102(2): 362-375.
- Mohammad Mehedi Hasan, Janeli Viil, Freddy Lättekivi, James Ord, Qurat Ul Ain Reshi, et al. (2020) Bovine Follicular Fluid and Extracellular Vesicles Derived from Follicular Fluid Alter the Bovine Oviductal Epithelial Cells Transcriptome. Int J Mol Sci 21(15).
- Pradeepthi Bathala, Zeinab Fereshteh, Kun Li, Amal A Al-Dossary, Deni S Galileo, et al. (2018) Oviductal extracellular vesicles (oviductosomes, OVS) are conserved in humans: murine OVS play a pivotal role in sperm capacitation and fertility. Mol Hum Reprod 24(3): 143-157.
- Al Dossary AA, Strehler EE, Martin-Deleon PA (2013) Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS One 8(11): e80181.
- Yuta Matsuno, Takuya Kanke, Natsumi Maruyama, Wataru Fujii, Kunihiko Naito, et al. (2019) Characterization of mRNA profiles of the exosome-like vesicles in porcine follicular fluid. PLoS One 14(6): e0217760.
- Anise Asaadi, Nima Azari Dolatabad, Hadi Atashi, Annelies Raes, Petra Van Damme, et al. (2021) Extracellular Vesicles from Follicular and Ampullary Fluid Isolated by Density Gradient Ultracentrifugation Improve Bovine Embryo Development and Quality. Int J Mol Sci 22(2).
- O Neil EV, Burns GW, Spencer TE (2020) Extracellular vesicles: Novel regulators of conceptus-uterine interactions? Theriogenology 150: 106-112.
- York Hunt Ng, Sophie Rome, Audrey Jalabert, Alexis Forterre, Harmeet Singh, et al. (2013) Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS One 8(3): e58502.
- Bidarimath M (2017) Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: A new paradigm for conceptus-endometrial cross-talk. Sci Rep 7: 40476.
- Keerthie Dissanayake, Monika Nõmm, Freddy Lättekivi, Yosra Ressaissi, Kasun Godakumara, et al. (2020) Individually cultured bovine embryos produce extracellular vesicles that have the potential to be used as non-invasive embryo quality markers. Theriogenology 149: 104-116.
- Edwin A Mellisho, Alejandra E Velásquez, María J Nuñez, Joel G Cabezas, Juan A Cueto, et al. (2017) Identification and characteristics of extracellular vesicles from bovine blastocysts produced in vitro. PLoS One 12(5): e0178306.
- Masood Abu-Halima, Sebastian Häusler, Christina Backes, Tobias Fehlmann, Claudia Staib, et al. (2017) Micro-ribonucleic acids and extracellular vesicles repertoire in the spent culture media is altered in women undergoing In Vitro Fertilization. Sci Rep 7(1): 13525.
- Gopichandran N, Leese HJ (2006) The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction 131(2): 269-277.
- Islam M Saadeldin, Su Jin Kim, Yoo Bin Choi, Byeong Chun Lee (2014) Improvement of cloned embryos development by co-culturing with parthenotes: a possible role of exosomes/microvesicles for embryos paracrine communication. Cell Reprogram 16(3): 223-234.
- Danilo Buca, Giuseppina Bologna, Alice D Amico, Sara Cugini, Francesca Musca, et al. (2020) Extracellular Vesicles in Feto-Maternal Crosstalk and Pregnancy Disorders. Int J Mol Sci 21(6).
- Manjot Gill, Carolina Motta-Mejia, Neva Kandzija, William Cooke, Wei Zhang, et al. (2019) Placental Syncytiotrophoblast-Derived Extracellular Vesicles Carry Active NEP (Neprilysin) and Are Increased in Preeclampsia. Hypertension 73(5): 1112-1119.
- Burton GJ, auniaux EJ (2015) What is the placenta? Am J Obstet Gynecol 213(4 Suppl): S6.e1, S6-8.
- Carlos Salomon, Miharu Kobayashi, Keith Ashman, Luis Sobrevia, Murray D Mitchell, et al. (2013) Hypoxia-induced changes in the bioactivity of cytotrophoblast-derived exosomes. PLoS One 8(11): e79636.
- Kshirsagar SK, Alam SM, Jasti S, Hodes H, Nauser T, et al. (2012) Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 33(12): 982-990.
- Sean L Nguyen, Soo Hyun Ahn, Jacob W Greenberg, Benjamin W Collaer, Dalen W Agnew, et al. (2021) Integrins mediate placental extracellular vesicle trafficking to lung and liver in vivo. Sci Rep 11(1): 4217.
- Samantha Sheller Miller, Kyungsun Choi, Chulhee Choi, Ramkumar Menon (2019) Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. Am J Obstet Gynecol 221(5): 502.e1-502.e12.
- Sarwat I Gilani, Tracey L Weissgerber, Vesna D Garovic, Muthuvel Jayachandran (2016) Preeclampsia and Extracellular Vesicles. Curr Hypertens Rep 18(9): 68.
- Tong M, Chamley LW (2015) Placental extracellular vesicles and feto-maternal communication. Cold Spring Harb Perspect Med 5(3): a023028.
- Carlos Salomon, Jennifer Ryan, Luis Sobrevia, Miharu Kobayashi, Keith Ashman, et al. (2013) Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One 8(7): e68451.
- Burton GJ, Fowden AL, Thornburg KL (2016) Placental Origins of Chronic Disease. Physiol Rev 96(4): 1509-1565.
- Kanada M, Bachmann MH, Contag CH (2016) Signaling by Extracellular Vesicles Advances Cancer Hallmarks. Trends Cancer 2(2): 84-94.
- Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science 367(6478).
- Khalid Al Nedawi, Brian Meehan, Johann Micallef, Vladimir Lhotak, Linda May, et al. (2008) Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 10(5): 619-624.
- Anoek Zomer, Carrie Maynard, Frederik Johannes Verweij, Alwin Kamermans, Ronny Schäfer, et al. (2015) In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161(5): 1046-1057.
- Gavin P Collett, Christopher W Redman, Ian L Sargent, Manu Vatish (2018) Endoplasmic reticulum stress stimulates the release of extracellular vesicles carrying danger-associated molecular pattern (DAMP) molecules. Oncotarget 9(6): 6707-6717.
- Noriyoshi Watanabe, Takeo Fujiwara, Tomo Suzuki, Seung Chik Jwa, Kosuke Taniguchi, et al. (2014) Is in vitro fertilization associated with preeclampsia? A propensity score matched study. BMC Pregnancy Childbirth 14: 69.
- Huang Doran I, Zhang CY, Vidal Puig A (2017) Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol Metab 28(1): 3-18.
- Mariëtte E G Kranendonk , Frank L J Visseren, Bas W M van Balkom, Esther N M Nolte t Hoen, Joost A van Herwaarden, et al. (2014) Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring) 22(5): 1296-1308.
- Beth S Holder, Clare L Tower, Karen Forbes, Melissa J Mulla, John D Aplin, et al. (2012) Immune cell activation by trophoblast-derived microvesicles is mediated by syncytin 1. Immunology 136(2): 184-191.
- Yu Zhaoer, Gao Mingming, Zhang Wei, Yao Dan, Qian Yating, et al. (2022) Extracellular vesicles for the treatment of preeclampsia. Tissue Cell 77: 101860.
- Jin Cai, Yu Han, Hongmei Ren, Caiyu Chen, Duofen He, et al. (2013) Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol 5(4): 227-238.
- Cha Han, Chenyu Wang, Yuanyuan Chen, Jiwei Wang, Xin Xu, et al. (2020) Placenta-derived extracellular vesicles induce preeclampsia in mouse models. Haematologica 105(6): 1686-1694.
- Carlos Salomon, Dominic Guanzon, Katherin Scholz-Romero, Sherri Longo, Paula Correa, et al. (2017) Placental Exosomes as Early Biomarker of Preeclampsia: Potential Role of Exosomal MicroRNAs Across Gestation. J Clin Endocrinol Metab102(9): 3182-3194.
- Angélique Bobrie, Marina Colombo, Sophie Krumeich, Graça Raposo, Clotilde Théry (2012) Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles 1.
- David W Greening, Shashi K Gopal, Rong Xu, Richard J Simpson, Weisan Chen, et al. (2015) Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol 40: 72-81.
- Robbins PD, Morelli AE (2014) Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 14(3): 195-208.
- Haiying Zhang, Daniela Freitas, Han Sang Kim, Kristina Fabijanic, Zhong Li, et al. (2018) Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 20(3): 332-343.
- Chris Gardiner, Dolores Di Vizio, Susmita Sahoo, Clotilde Théry, Kenneth W Witwer, et al. (2016) Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles 5: 32945.
- Bruno Costa Silva, Nicole M Aiello, Allyson J Ocean, Swarnima Singh, Haiying Zhang, et al. (2015) Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17(6): 816-826.
- Ayuko Hoshino, Bruno Costa-Silva, Tang-Long Shen, Goncalo Rodrigues, Ayako Hashimoto, et al. (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527(7578): 329-335.
- Guay C, Regazzi R (2017) Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab 19 Suppl 1: 137-146.
- Oscar P B Wiklander, Joel Z Nordin, Aisling O Loughlin, Ylva Gustafsson, Giulia Corso, et al. (2015) Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4: 26316.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...