Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Article(ISSN: 2638-6070) 
Hypocholesterolemic Effect of Pumpkin Kernel and Defatted Meal in Mice Fed a High-Cholesterol Diet
Volume5- Issue1Al-Surmi NY1*, Al Anoos EM2 and Hasanin HA3
- 1Genetic Resources Center, Faculty of agriculture, Sana’a University, Yemen
- 2Department of food industries, Faculty of Agriculture, Damietta University ,Egyt
- 3Food Technology Research Institute, Agriculture Research Center, Giza, Egypt
Received:June 19, 2023; Published:August 28, 2023
*Corresponding author: Al Surmi NY, Genetic Resources Center, Faculty of agriculture, Sana’a University, Yemen
DOI: 10.32474/SJFN.2020.03.000202
Abstract
This research evaluated the hypocholesterolemic impacts of pumpkin kernel and defatted meal in mice using three different types of pumpkin seeds: two Egyptian varieties (Kafr Saad and Kafr Al-Battikh) and a Chinese variety called Hongli. Six groups of mice were formed: a normal control group (NC), a hyperlipidemic control group (HC), a group of mice given defatted pumpkin meal (20 and 40%), and a group of mice given pumpkin kernels (20 and 40%). According to the results, pumpkin seeds at 20 and 40 % had a substantial impact on decreasing cholesterol more effectively than defatted flour, with the high cholesterol control group’s total cholesterol dropping from 135 mg/dl to 106.43 mg/dl when the seeds at 40 % were consumed. These results stimulate the use of pumpkin seeds to prevent high cholesterol and its serious consequences for human health.
Introduction
The gourd squash known as pumpkin (Cucurbita mixta) is a member of the Cucurbitaceae family [1,2]. There are over 80 genera and about 800 species in the Cucurbitaceae family [3]. The pumpkin plant is a perennial that grows leafy green crops and can grow up to 12 meters tall [4]. All of a pumpkin seed’s components including the hard shell, seeds, and even the flowers and leaves, are edible. Pumpkin seeds are useful in a variety of ways. One can boil, bake, steam, or roast ripe pumpkin fruit. Pumpkins can be consumed in a variety of ways, including raw or cooked as a vegetable, frozen or preserved [5]. Pumpkin flour has been reported to be used as a substitute for grain flour in baked products, soups, sauces, instant noodles, and dressings, and as a natural coloring in pasta, flour mixes and candies. Pumpkin seeds, commonly called ‘pepitas’, are flat and covered with yellow and white shells [6]. Although they also contain plant compounds, pumpkin seeds have significant antinutritional effects in certain essential amounts [7].
These substance include phytates, nitrates, cyanides, oxalates, and more. In various cultures, pumpkin seeds are eaten directly as a snack [4]. According to Murkovic et al. [8], roasting the seeds increases their nutritional value. When roasted seeds were compared to fresh seeds, a higher ratio of sterols and vitamin E was found. Pumpkin seeds contain high amounts of protein (25-52%). Bombardelli and Morazzoni et al. [9]. The oil content is as high as 40-60% [10]. Up to 60.8% of this consists of the fatty acids oleic (up to 46.9%), linolenic (up to 40.5%), palmitic and stearic (up to 17.40%). 0.60 to 0.75 grams of monounsaturated to polyunsaturated acids. The content of phytosterols, squalene and chlorophyll pigments is about 1% each. There are also phytosterols in free and bound forms. The content of minerals is 4-5%, including selenium, zinc, calcium, copper, iron, manganese, phosphorus, and potassium. 30% pectin content [9].
In Egypt, pumpkin seeds are an interesting alternative to Western popcorn eaten as part of the culture [6]. In many civilizations of the world, people eat pumpkin seeds raw as a snack. After salting and roasting, the seeds are very popular, especially in Arab countries [11]. In Austria, pumpkins are mainly grown for the production of salad oil or edible pumpkin seeds [12]. Pumpkin seeds (Cucurbita maxima) have long been used for medicinal, medicinal, and clinical purposes. They contain a number of useful functional elements. Pumpkin seed oil is used as a nerve tonic as well as a safe anthelmintic and diuretic [13, 14]. Pumpkin seed oil is a powerful antioxidant [15]. Health benefits include preventing enlargement and shrinkage of the prostate, delaying the development of hypertension, reducing pain associated with high cholesterol and arthritis, reducing bladder and urethral pressure, improving bladder compliance, reducing diabetes by promoting hypoglycemia effect and a reduced incidence of gastrointestinal diseases, enterocolitis, breast, lung and colon cancer [15,16]. In many countries, pumpkin seeds are used extensively for protein or oil production [17].
Pumpkin seeds are used in Austria, Slovenia, Hungary and Serbia to produce high quality salad oil [18]. Jams, marmalades and preserves can add flavor to dishes and are an effective way to use fruits that cannot be preserved or frozen [19]. In addition, pumpkin can be used as a natural coloring and processed into flour that has a longer shelf life and is used as a substitute for wheat flour in baked goods, soups, sauces, instant noodles, and dressings [20].
Material and Methods
Pumpkin Seed Samples
Samples of pumpkin seeds were used in this investigation, including two Egyptian cultivars-Kafr Saad and Kafr El-batikh-as well as one imported cultivar from China, which was purchased at a local market in Cairo City during the growing season. The seeds were freed from foreign contaminants, manually dehulled, and then crushed using an electronic grinder before being stored at -20 °C for the upcoming examination.
Analytical Methods
Pumpkin Oil Samples and Defatted Oil Cake
The total lipids in ground pumpkin seeds were extracted using a 2:1 v/v mixture of chloroform and methanol. The mixture was mechanically shaken for 16 hours, after which it was filtered. The filtrate was filtered since being washed three times with a chloroform and methanol solution. The resultant filtrate was allowed to dry outside at a temperature of around 25 °C. The recovered lipids were filtered over anhydrous sodium sulphate and kept at -20°C for further analysis in dark brown glass bottles. After being defatted and dried, the flour was crushed to pass through a 70 mesh screen and kept at 0°C for further examination [21].
Preparation of Pumpkin Defatted seed Meal
The pumpkin kerenls were dried before lipid extraction. Lipid extraction from the dry kernels was carried out by n- hexane extraction under the operating conditions specified in IUPAC methods no. 1.121.(1987) [22].
Gross Chemical Composition
Moisture, crude oil, total nitrogen, ash content and crude fiber of pumpkin seeds kernels were determined as outlined in A.O.A.C Carbohydrate was calculated by difference(1997) [23].
Animals Experimental Design
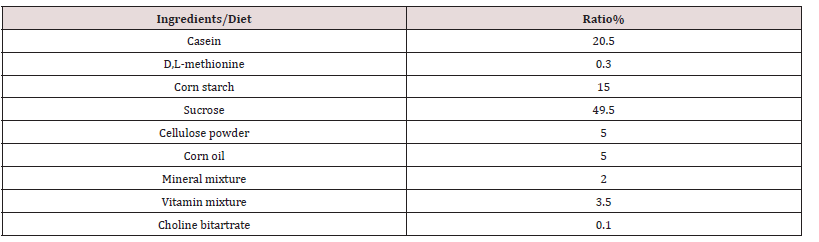
Adult female Swiss common bred albino mice purchased from Theudorus Bilharz Institute, Giza, Egypt, with an average body weight of 25 to 30 g were used. Mice were randomly divided into six groups of rather similar total weight. Each group consisted of seven mice. The mice in each group were assigned to the corresponding experimental diet and were housed individually in cages in a controlled environment. Diets and water were supplied ad libitum throughout the study.
Diets
Rich Cholesterol Diet (RCD): Basal Diet + 2% cholesterol Mice
groups fed during experimental time as follows:
Group I. Normal mice fed basal diet (negative control). for 30
days.
Group II. Normal mice fed basal diet+2%cholesterol for 30
days.
Groups III. Normal mice fed basal diet+2% cholesterol + %
Kafr Saad pumpkin defatted meal for 30 days.
Group IV. Normal mice fed basal diet+2%cholesterol+ % Kafr
Saad pumpkin defatted meal for 30 days.
Group V. Normal mice fed basal diet+2%cholesterol+ % Kafr
Saad pumpkin whole kernel for 30 days.
Group VI. Normal mice fed basal diet+2%cholesterol+ % Kafr
Saad pumpkin whole kernel for 30 days.
Blood Samples
Mice of each group were sacrificed after the end of treatment period, and the blood samples were collected and subjected to different laboratory assay, in which small part was separated for hematological determinations. The other blood portion was lifted to clotting, centrifuged at 3000 rpm for 15 minutes to separate serum. It was freshly used in the determination of some parameters and the other was quickly frozen till the biochemical assay was carried out.
Triglyceride determination
Triglycerides were enzymatically determined according to the method of Fossati and Principe et al., [24]. The developed color was measured at 546 nm against dist. H O as a blank sample. Calculation
Triglyceride concentration
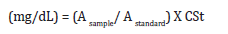
Where,
Asample: Absorbance of sample.
Astandard: Absorbance of standard.
CSt: Cholesterol (200 mg/dL).
Total cholesterol determination
Total cholesterol was determined in serum samples according to the method of Richmond et al., [25]. The developed color was measured at 500 nm against dist. H O as a blank sample. Calculations
Concentration of concentration (mg/dL) = (Asample/ Astandard) X CSt
Where,
Asample:Absorbance of sample.
Astandard: Absorbance of standard.
CSt: Cholesterol (200 mg/dL).
Estimation of HDL cholesterol in serum
HDL cholesterol concentrations were measured colorimetrically with commercially available kits (HDL-cholesterol test, ELITECH diagnostics French), According to the quantitative estimation of HDL cholesterol was done using HDL cholesterol precipitating reagent in combination with enzymatic colorimetric assay kit for total cholesterol. Chylomicrons, very low density lipoprotein (VLDL) cholesterol, and low density lipoprotein (LDL) cholesterol fractions are precipitated from serum or plasma by means of phosphotungstic acid and magnesium ions, according to Lopes-Virella et al., [26] After centrifugation, high density lipoprotein HDL) cholesterol is then determined in the supernatant fluid. using a cholesterol regent and the derived dilution factor in the calculation (Table 1).
Concentration of concentration (mg/dL) = (Asample/ Astandard) X CSt
Where,
Asample:Absorbance of sample.
Astandard: Absorbance of standard.
CSt: Conc standard (200 mg/dL).
Estimation of LDL and VLDL cholesterol in serum:
The concentration of LDL cholesterol was calculated as the
equation
of Fried Ewald et al as follows:
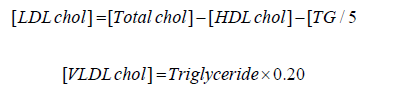
Results
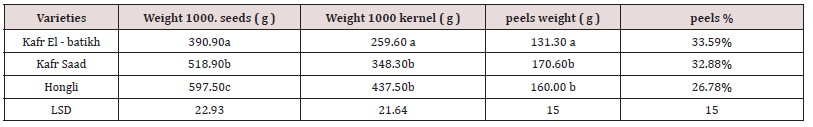
Weight of seeds , kernel and peels
The data in Table 2 indicated that there were significant differences between the studied cultivars in their contents of seeds. However, KafrSaad and Hongli cultivars consider a good source of seeds among the studied cultivars, while Kafr El-batikh cultivar is the least in their content of seeds (Table 2).
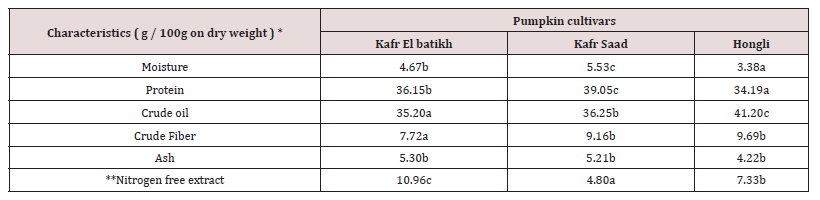
Gross chemical composition
(Table 3) shows the approximate chemical make-up of pumpkin seeds. The results of Onimawo et al., [27-29] who reported that the moisture content was 3.7, 5.11, and 5.20%, respectively, were in agreement with the moisture content range of 3.38 to 5.53%. In contrast to local varieties, the imported Chinese Hongli cultivar had a somewhat lower moisture content. Also, the lower moisture content is crucial for maintaining quality while in storage. The range of the pumpkin seeds under investigation’s protein content was 39.05%. (on dry weight basis). The calculated protein content values agreed with data from Alfawaz et al., [30-33]. According to Table, the examined pumpkin seeds’ crude oil content ranged from 35.20 to 41.20%. The examined pumpkin varieties’ seeds had a high oil content. Similar findings were also reported by Gohari Ardabili et al., [29, 34,35 ]. Data in Table 2 revealed that, Hongli pumpkin cultivar seeds have the highest value of crude fiber (9.69%) followed by Kafr saad cultivar (9.16%) finally, Kafr El-batikh cultivar (7.72%).
Values with different subscripts on the same column are significant (p> 0.05)
*calculated by differences*On dry weight bassis%
** Calculated by difference
Similar results for crude fiber was reported by Abd El- Ghany et al., [28]. On other hand, the data revealed that, the Nitrogen free extract was ranged from 4.80 to 10.96% in the studied pumpkin seeds Moreover, similar results were reported by Hegazy and El Kinawy et al., [32,34,36]. The ash content was ranged from 4.22 to 5.30 % as indicated in Table 1. Similar results for ash content of various cultivars of pumpkin seeds were reported by Gohari Ardabili et al., [29,32,34].
Nutritional Utilization of Pumpkin Seeds
Hypercholesterolemia is a major cause of cardiovascular disease (CVD), such as atherosclerosis and coronary heart disease. CVD is the most common cause of mortality and morbidity worldwide. Hyperlipidemia refers to either increased serum total cholesterol level or serum triglyceride level or both. Hyperlipidemia or hypercholesterolemia leads to the development of atherosclerosis and later leads to the progression of Coronary Heart Disease (CHD), which will cause cardiovascular morbidity and mortality. Although several factors, such as cigarette smoking, high-fat diet, high blood pressure, physical inactivity, age, and heredity have significant roles in causing CVD, high blood cholesterol is mainly responsible for the onset of CVD [37]. Lowering serum cholesterol levels by drug or dietary interventions could reduce the risk of CVD. Therefore, it is worthwhile to develop new safe and effective cholesterol-lowering agents from natural products. Pumpkin seed contain phytosterol, which help for reduce hypercholesterolemia. The presence of a high amount of cholesterol in the diet has been demonstrated to elevate total cholesterol and may increase the risk of cardiovascular complications. Agents that can lower serum cholesterol and scavenge or inhibit free radical formation have gained wide therapeutic value. Great efforts have been made to reduce the risk of cardiovascular disease (CVD) through the regulation of cholesterol, and the ther apeutic benefits of plants have been the focus of many extensive dietary studies [38].
Serum Lipid Profile
In the present study, the lipid-lowering effect of pumpkin defatted meal and whole seed in mice fed a high- cholesterol diet was investigated. Kafr Saad variety was chosen for the experiment because it has the highest percentage of mono and polyunsaturated fatty acids , the active ingredients responsible for its biological effects. Mice were fed a high cholesterol diet for four weeks. Notably, the mice fed with the dietary cholesterol showed a significant increase in serum triglycerides (TG), total cholesterol (TC) and low density lipoprotein (LDL) levels compared to those of the normal group (normal diet). On the other hand, the mean of HDL levels were decreased as shown in Table 4. Serum cholesterol was elevated significantly in the high cholesterol diet group compared with the control group. In the addition, mice treated with pumpkin defatted meal 20 and 40% mice group had no significant difference compared to high cholesterol diet group. While there was significant lowering in 20 and 40% whole seed groups and 21.16%); respectively compared to that of the high cholesterol-diets group. The results of the Table 1 added that, the mean levels of triglycerides were significantly improved by decrease (6.30%) in mice treated with 40% pumpkin defatted meal, and 5.54 and 17.44% in mice treated with 20 and 40% whole seed groups respectively . LDL was significantly decreased by (19.91 and 35.64%) in the mice treated with 20 and 40% whole seed groups respectively compared to high cholesterol diet group. Simultaneously, the mean levels of HDL were significantly increased by (13.03%) of mice treated 40% whole seed group, when the levels were compared those with the mice fed with high cholesterol diet. VLDL was significantly decreased by (17.45%) in the mice treated with 40% whole pumpkin seed groups. The obtained results were in agreement with that reported by Al showayman et al.[39-41].
Table 4: Effect of pumpkin defatted powder and kernels on Serum lipid profile in mice fed high cholesterol diet.
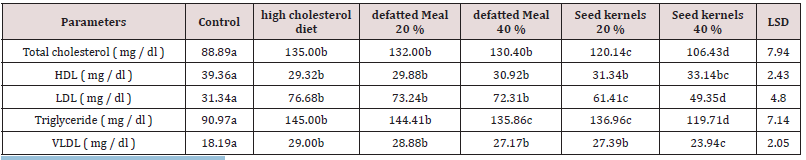
Values were expressed as mean ( n = 7 )
Means significantly different from Hypercholestrolemi group at P < 0.05.
References
- Borhade S (2012) Extraction and characterization of pumpkin (Cucurbita mixta) seed oil. Life sciences Leaflets 29: 45-49.
- Kim MY, Kim EJ, Kim YN, Choi,C and Lee BH (2012) Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutrition research and practice 6(1): 21-27.
- Giampan J, Rezende J and Silva R(2007) Reaction of cucurbits species to infection with Zucchini lethal virus. Scientia Horticulturae 114(2): 129-132.
- Dietmar F (2005) Extract of Pumpkin Seeds Suppresses Stimulated Peripheral Blood Mononuclear Cell Invitro. American Journal of Immunology 1(1): 6-11.
- Figueredo E, Minguez A and Vidarte LL (2000) Allergy to pumpkin and cross-reactivity to other Cucurbitaceae fruits. Journal of Allergy Clinical Immunology 106(2): 402-403.
- Abdel Rahman MK (2006) Effect of pumpkin seed (Cucurbita pepo L) diets on benign prostatic hyperplasia (BPH): Chemical and morphometric evaluation in rats. World J of Chemistry 1(1): 33-40.
- Omorayi FO and Dilworth L (2007) Antinutritional factors, Zinc, Iron, and Calcium in some Cariggean tuber crops and effects of boiling or roasting. Nutrition and Food Sciences 37(1): 8-15.
- Murkovic M, Piironen V, Lampi AM, Tanja K and Gerhard S (2003) Changes in chemical composition of pumpkin seeds during the roasting process for production of pumpkin seed oil (Part 1: non-volatile compounds). Food Chemistry 84(3): 359-365.
- Bombardelli E and Morazzoni P (1997) Curcubita pepo L itoterapia. 4: 291-302.
- Nakic SN, Rade D, Skevin D, Strucelj D, Mokrovcak Z, et al. (2006) Chemical characteristics of oils from naked and husk seeds of Cucurbita pepo L. Eur J Lipid SciTechnol 108(11): 936-943.
- Al-Khalifa AS (1996) Physicochemical characteristics, fatty acid composition and lipoxygenase activity of crude pumpkin and melon seed oil. J Agric Food Chem 44(4): 964-966.
- Murkovic M and Pfannhauser W (2000) Stability of pumpkin seed oil. Eur J Lipid Sci Technol 102(10) : 607-611.
- Younis YMH, Ghirmay S and Al-Shihry SS (2000) African Cucurbita pepo L: Properties of seed and variability in fatty acid composition of seed oil. Phytochem 54(1): 71-75.
- Ghani A (2003) Medicinal Plants of Bangladesh. Asiatic Society of Bangladesh, Dhaka.
- Stevenson DG, Eller FJ, Wang L, Jane JL, Wang T, et al. (2007) Oil and Tocopherol Content and Composition of Pumpkin Seed Oil in 12 Cultivars. J Agric Food Chem 55(10): 4005 -4013.
- Caili F, Huan S and Quanhong L (2006) A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods for Human Nutr 61(2): 73-80.
- Tsaknis J, Lalas S, Lazos ES(1997) Characterization of crude and purified pumpkin seed oil. Grasas Aceites 48(5): 267-272.
- Pericin D, Krimer Trivic VS and Radulovic L (2009) The distribution of phenolic acids in pumpkin’s hull less seed skin, oil cake meal, dehulled kernel and hull. Food Chem 113(2): 450-456.
- Egbekun MK, Nda Suleiman EO and Akinyeye O (1998) Utilization of fluted pumpkin fruit (Telfairia occidentalis) in marmalade manufacturing. Plant Foods for Human Nutr 52(2): 171-176.
- See EF, Nadiah WA and Aziah AA (2007) Physico Chemical and Sensory Evaluation of Breads Supplemented with Pumpkin Flour. ASEAN Food Journal 14 (2): 123-130.
- Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226(1): 497-509.
- IUPAC (International union of pure and applied chemistry) (1987): Standard methods for the analysis of oils, fat and derivatives; 7th revised and enlarged edn., edited by C. Paquat and A.Hautfenne, Blackwell Scientific, London.
- AOAC (1997) Official Methods of Analysis of AOAC International, (16th edition, 3rd Revision), AOAC International, Suite 500, 481 North Frederick Avenue, Gaithersburg, Maryland, USA.
- Fossati P and Principe L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clinical Chemistry 28(10): 2077-2080.
- Richmond W (1973) Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clinical Chemistry 19(12): 1350-1356.
- Lopes Virella MF, Stone P, Ellis S and Coiweil JA (1977) Cholesterol Determination in High-Density Lipoproteins Separated by Three Different Methods. Clin Chem 23(5): 882-884.
- Onimawo IA, Nmerole EC, Idoko PI and Akubor PI (2003) Effects of fermentation on nutrient content and some functional properties of pumpkin seed (Telfaria occidentalis). Plant Foods for Human Nut 58(3): 1–9.
- Abd El-Ghany MA, Hafez DA and El Safty SMS (2010) Biological study on the effect of pumpkin seeds and zinc on reproductive potential of male rats. Annual Scientific Conf 2384-2404.
- Gohari Ardabili A, Farhoosh R, Haddad Khodaparast MH (2011) Chemical composition and physicochemical properties of pumpkin seeds (Cucurbita pepo subsp. Pepo var. Styriaka) grown in Iran. Journal of Agricultural Science and Technology 13(7): 1053-1063.
- Alfawaz MA (2004) Chemical composition and oil characteristics of pumpkin (Cucurbita maxima) seed kernels. Food sci & agric res center 5-18.
- Badr SEA, Shaaban M, Elkholyc YM, Helal MH, Hamza AS, et al. (2010) Chemical composition and biological activity of ripe pumpkin fruits(Cucurbita pepo L) cultivated in Egyptian habitats. Natural Product Research 25(16): 1524-1539.
- Rodríguez Miranda J, Hernández Santos B, Herman Lara E, Vivar Vera MA, Carmona García R, et al. (2012) Physicochemical and functional properties of whole and defatted meals from Mexican (Cucurbita pepo) pumpkin seeds. International Journal of Food Science & Technology 47(11): 2297-2303.
- Steiner Asiedu M, Nuro Ameyaw P, Agbemafle I, Hammond SH and Tano Debrah K (2014) Nutrient Composition and Protein Quality of Four Species of the Curcubitaceae Family.
- Jafari M, Goli SAH, Rahimmalek M (2011) The chemical composition of the seeds of Iranian pumpkin cultivars and physicochemical characteristics of the oil extract. Eur J Lipid Sci Technol 114(2): 1-7.
- Nyam KL, Tan CP, Lai OM, Long K and Che Man YB (2009) Physicochemical properties and bioactive compounds of selected seed oils. Food Sci and Technol 42(8): 1396-1403.
- Hegazy EM, El Kinawy OS (2011) Characteristics, of Pumpkin and Bottle Gourd in Egypt and Potentially their Contamination by Mycotoxins and Mycotoxigenic Fungi. Journal of American Science 7(9): 615-622.
- Yokozawa T, Ishida A, Cho EJ, Nakagawa T(2003) The effects of Coptidis rhizoma extract on a hypercholesterolemic animal model. Phytomedicine 10(1):17-22.
- Zhang HW, Zhang YH, Lu MJ (2007) Comparison of hypertension dyslipidaemia and hyperglycaemia between buckwheat seed consuming and nonconsuming Mongolian-Chinese populations in Inner Mongolia, China. Clin Exp Pharmacol Physiol 34(9): 838- 844.
- Al-showayman SIA (2010) The effect of pumpkin seed feeding on the serum lipid profile and c-reactive proteinin atherogenic rats. King Saud University Deanship of Gaduate Studies 1-99.
- Landeka I, Dikic D, Radisic I, Teparic R, Bacun Druzina V, et al.(2011) The effects of olive and pumpkin seed oil on serum lipid concentrations. Journal of Food Technology, Biotechnology and Nutrition 6(1-2): 56-63.
- El-Adawy TA and Taha KM (2001) Characteristics and composition of different seed oils and flours. Food chem 74(1): 47-54.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...