Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4706
Research Article(ISSN: 2637-4706) 
Antioxidant Properties and Antibacterial Activities of Leptadenia hastata Leaves Extracts on Staphylococcus aureus Volume 1 - Issue 5
Isaac John Umaru1,2*, Fasihuddin A Badruddin1 and Hauwa A Umaru3
- 1Faculty of Resource Science and Technology Universiti Malaysia Sarawak, Malaysia
- 2Department of Biochemistry, Federal University Wukari, Taraba State, Nigeria
- 3Department of Biochemistry, Moddibo Adama University, Yola Adamawa State, Nigeria
Received: June 10, 2018; Published: June 18, 2018
Corresponding author:Isaac John Umaru, Medicinal Biochemistry Federal University, Wukari Taraba State, Malaysia
DOI: 10.32474/DDIPIJ.2018.01.000122
Abstract
Leptadenia hastata extracts were evaluated for antibacterial activity and antioxidant properties. The leaf of the plant was extracted with n-hexane, dichloromethane, ethyl acetate, chloroform and methanol to give respective extracts. The Antibacterial activity against Staphylococcus aureus, was determined by disc diffusion method. Antioxidant activity was assayed by the DPPH radical scavenging activity mechanism. The results showed that of n-hexane, ethyl acetate, dichloromethane, chloroform and methanol extracts of Leptadenia hastata, methanol extract and chloroform extracts displayed more activity with 1.10 ± 0.10ab and 0.97 ± 0.06ab than others at 25-1000ppm/desk of the extracts tested. The most antioxidant activities against DPPH were displayed by the hexane and methanol extracts of Leptadenia hastata leaf, exhibiting IC50 (63.44μg/mL) and IC50 (46.80μg/mL) respectively, followed by chloroform, dichloromethane and lastly ethyl acetate crude extract. The present results showed potential of the medicinal plant used by traditional herbal medical practitioners as natural anti-microbial, antioxidant and potentially antiinflammatory agents. The results confirm that Leptadenia hastata leaves extracts can be used as a source of drugs to fight infections caused by susceptible bacteria.
Keywords: Staphylococcus aureus, Leptadenia hastata, 2; 2-diphenyl-1-pycryl-hydrazyl
Introduction
In recent years, there has been an increasing awareness about the importance of medicinal plants. Drugs from these plants are easily available, inexpensive, safe, efficient, and rarely accompanied by side effects. Plants which have been selected for medical use over thousands of years constitute the most obvious starting point for new therapeutically effective drugs such as anticancer drugs [1] and antimicrobial drugs [2]. Recently, medicinal plants usage has increased in spite of the advances made in the field of chemotherapy. The reasons proposed [3] are the use of medicinal plants as materials for the extraction of active pharmacological agents. There is also the increased use of medicinal plants in industrialized countries for medicinal purposes. Leptadenia hastata despite the extensive uses, there have been only limited attempts to explore the biological activities of the plants in relation to their medicinal uses. Here, we present data on antibacterial activities and antioxidative potentials of the different extracts obtained from n-hexane dichloromethane, ethyl acetate, chloroform and methanol. Leptadenia hastata (Pers.) Decne, which belongs to the family Asclepiadaceae, is a wild plant used as vegetable by many African populations and medicine due to its nutritive and therapeutic properties for the treatment of wounds and stomach upset conditions in children [4,5]. The plant Leptadenia hastata is an edible non-domesticated valuable herb with creeping latex stems, glabescent leaves, glomerulus and recemes flowers as well as follicle fruits. It is typically grown in tropical dry land in sand soil [6].
The plant is commonly used in the north Nigeria as spices and used as sauces [7], as a vegetable in Niger republic [8-11]. Local healers also use the plant for hypertension, catarrh and skin diseases [12]. In certain areas of West Africa, breeders claimed the antifertility effect of their animals after consumption of Leptadenia hastata leaf [13] this paper presents an investigation of Leptadenia hastata (Pers) Decne, antibacterial and Antioxidant. Which is one of the contributing factors responsible for the antimicrobial activity of the extracts of the leaves on Staphylococcus aureus species? The findings in this study may contribute to the present literature in understanding the bioactive value of the crawling plant Leptadenia hastata.
Material and Method
Sample Collection
Leptadenia hastata leaves: freshly leaves of Leptadenia hastata were collected from the uncultivated farmland of the Federal University Wukari Taraba State, Nigeria and was authenticated at Ahmadu Bello University Zaria and Voucher No PU: 2 ABU Herbarium No 900220. The plant Leptadenia hastata (yadiya) was dried under room temperature.
Preparation of Samples
Fresh leaves of the plant Leptadenia hastata was washed with distilled water to remove the soil and dust particles, they are thoroughly air dried and powdered using laboratory grinder machine (FGR-350, Quest Scientific). Extraction using hexane by placing 150g of the powdered samples into an Erlenmeyer flask and hexane three times the weight of the extracts was added, the solution was covered and shaken at an interval of an hour and then allowed at room temperature to stand for 7days. The mixture was then filtered using Whatman filter paper No.4 the residue was reextracted with fresh hexane for another 72 h and filtered. Both extracts were combined and concentrated with a rotary evaporator (Heidolph Laborota 4000 efficient) under reduced pressure toobtain the hexane crude extract. The residues were re-extracted using a similar procedure with dichloromethane (CH2CLl2), followed by ethyl acetate (C2H5COOH), chloroform (CHCL3), and methanol (MeOH) to obtain dichloromethane, ethyl acetate, chloroform and crude extracts, respectively. The dry weight and yield of each crude extracts were determined. It was then stored under a frozen condition until required.
DPPH (2, 2-diphenyl-1-pycryl-hydrazyl) Free Radical Scavenging Assay
The free radical scavenging assay of compound 2, 2-diphenyl- 1-pycryl-hydrazyl (DPPH) was used to evaluate the antioxidant properties of the crude extract and the essential oil. The measurement was based on the method described by [14]. The sample was prepared by diluting 6 mg of crude extract into 6 mL of methanol, producing a concentration of 1000 μg/mL. The stock solution was sonicated to ensure the homogeneity of the sample. Five other concentrations were prepared at 10, 50, 100, 500 and 1000 μg/mL, diluted from the 1000 μg/mL stock solution. Sample of 5000 μg/mL was prepared separately by diluting 25 mg of crude extract into 5mL of methanol. Approximately 3 mL of 0.1 mM solution of 2, 2 -diphenyl-1-pycrylhydrazyl (DPPH) in methanol was each added into six series of prepared concentrations (10, 50, 100, 500, 1000 and 5000 μg/mL) of sample solutions (1mL). Analysis was done in triplicate. The solution was mixed vigorously and left to stand at room temperature for 30 minutes in the dark after which its absorbance was measured spectrophotometrically at 517nm using Jasco ultra violet spectrophotometer model V-630. Methanol was used as blank (only methanol) and negative control (1mL methanol mixed with 3mL DPPH), while ascorbic acid (vitamin C) as the standard. The concentration of the sample required to inhibit 50% of the DPPH free radical was calculated as IC50 and the value was determined using Log dose inhibition curve which performed by using PRISM version 3.02 software, based on the calculated values of the DPPH scavenging activity (%) of the sample [15]. DPPH scavenging activity (%) was calculated with formula = A0-A1 / A0 x 100, where A0 was the absorbance of the control, while A1 was the absorbance in presence of the sample.
Preparation of Test Samples
The crude extracts of Leptadenia hastata was used in antibacterial assay namely the hexane, dichloromethane, ethyl acetate, chloroform and methanol crude extracts. The crude extracts were tested by disc diffusion method on nutrient agar medium as described by Ram Kumar & Pranay [16]. The plant exactly 5mg of each crude sample was dissolved homogeneity in 5mL of methanol giving a stock solution of 1000 μg/mL. Lower concentration of 25, 50, 100, 250, 500, and 1000ppm, i.e. six different volumes from the stock solution were taken for the studies.
Preparation of Agar Plates
Preparation of agar plates was performed based on method described by Ram Kumar & Pranay [16]. Nutrient agar was prepared according to manufacturer’s instruction with 14g of dried agar dissolved in 500 mL distilled water. The agar solution was heated until boiling followed by sterilization in autoclave at 121°C. The agar solution was then poured into a sterile petri plate and allowed to cool down and forming a gel. The plate was divided into eight sections by making a line marking on the outside surface of the plate. The six sections were for each test samples namely the 25ppm, 50ppm,100ppm, 250ppm, 500pp and 1000ppm samples, tetracycline 30μg (positive control) and methanol (negative control). The plate was sealed using parafilm and keep chilled at 4°C upon bacteria inoculation.
Preparation of Bacteria Broth v
The selected bacteria were used to evaluate the antibacterial activity of the crude extracts of Leptadenia hastata, Staphylococcus aureus (ATCC©25923 were obtained from the stock culture provided by Virology Laboratory, Universiti Malaysia Sarawak. The nutrient broth was prepared according to manufacturer’s instruction, with 2.6g of the dried broth dissolved in 200 mL distilled water followed by sterilization in autoclave at 121°C. The bacterial was sub-cultured in a 10 mL of broth, each in universal glass vail bottle for 16 hours inside an incubator equipped with shaker at 37°C [17]. After 16 hours’ incubation, turbidity (optical density/OD) of the bacterial broth was measured by using UV mini spectrophotometer (model 1240 of Shimadzu brand), comparable to that of nutrient broth standard tube for further use [18]. The measurement of the optical density was performed at wavelength 575nm and the bacterial broth was ready to be used when its turbidity was between OD 0.6 to 0.9. Nutrient broth was used to adjust the turbidity until the desired value was obtained.
Plate Inoculation
Inoculation of the bacteria was carried out in a biohazard cabinet and the procedure was based on method described by Ram Kumar & Pranay [16]. Approximately 1mL of the ready bacterial broth were transferred into mini centrifuge tubes. A sterile cotton swap was dipped into the mini centrifuge tube containing bacteria broth and streaked over entire of the agar plate surface, performed in 4 different directions. The agar plate was then left for 5-10 minutes before applying the test samples. The disc used was 6 mm diameter. A volume of 10 μL of the test samples of concentration 10, 25, 50, 100, 250, 500 and 1000μg/mL were each pupated onto the discs and placed onto the agar plate by using sterile forceps and gently pressed to ensure contact. Next to be placed on the agar plate was the disc pupated with methanol as negative control, followed by 30μg of tetracycline as standard antibacterial agent (positive control). The plates were left at room temperature for 10 minutes to allow the diffusion of the test samples and the standards into the agar. Each crude extract was tested in triplicate for the bacterium used. The plate samples were then incubated at 37°C for 24 hours before the inhibition zone around every sample disc being examined. The inhibition zone was measured in diameter (mm) to indicate the presence of antibacterial activity for each sample, as compared to the positive control.
Statistical Analysis
Statistical analysis for antibacterial activities was performed using SPSS programme. The IC50 for DPPH free radical scavenging assay was statistically determined using Log dose inhibition curve in PRISM programme.
Calibration equation = hillslope value X - bottom value
% Radical scavenging activities)=[1 - Abs sample - Abs blank]/[Abs control x 100]
Results and Discussion
Results
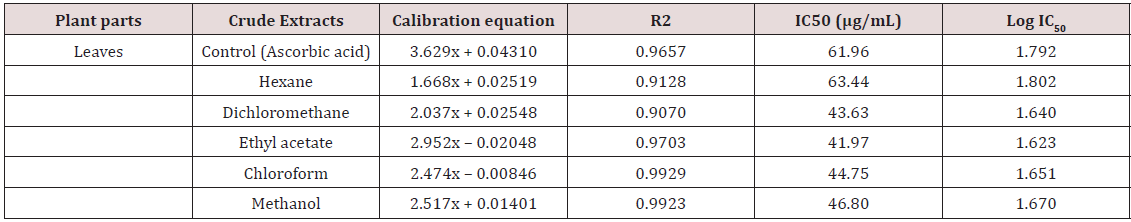
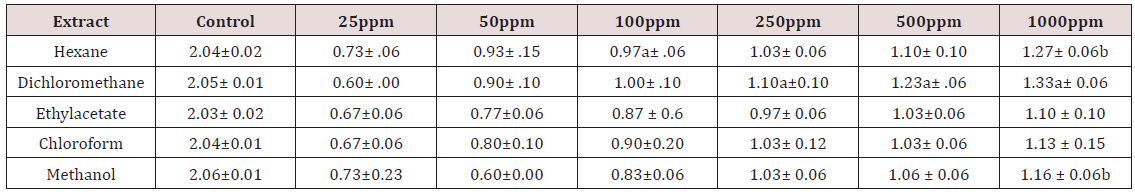
The development of antibacterial resistance to presently available antibiotics has led to the search for new antimicrobial agents [19]. Due to the problem of microbial resistance to antibiotics, attention is given toward biologically active components isolated from plant species commonly used as herbal medicine, as they may offer a new source of antimicrobial activities [20]. Our search for antioxidant potential and antibacterial bioactivity from tropical medicinal plant revealed the antioxidant and antibacterial activity of five different solvent leaf extracts of Leptadenia hastata. Results of antioxidant and antibacterial tests of the plant extracts are as listed in Tables 1 & 2 above.
Values are Mean ± SD for three determinations
aSignificantly (p< 0.05) higher compared to different extract at the same concentration
bSignificantly (p< 0.05) lower compared to the control.
Discussion
According to the results of disc diffusion assay, this leaf of the plant Leptadenia hastata has active compounds that are effective for the prevention of infections caused by Staphylococcus aureus. There are a number of factors that could influence the results of the disc diffusion assay. Firstly, the diameter of the zones is affected by the rate of diffusion of the antimicrobial compound [21,22] and thus may not exactly represent the potency of the extract’s antimicrobial activity. Where studies of plant extracts are concerned, the disc preparation technique could present with another problem wherein the extract was not properly and evenly impregnated into the paper discs. Another important factor is the standardization of the inoculum size to 0.5 McFarland turbidity. This inoculum size is important to ensure confluent or almost confluent lawn growth as a smaller inoculum size (such that single colonies are seen) may produce falsely large inhibition zones while a bigger inoculum size (thick bacterial lawn) may produce falsely smaller zones instead [23]. However, in the assay against Leptadenia hastata leaf extracts, i.e. n-hexane dichloromethane, ethyl acetate chloroform and methanol it displayed progressive activity at 25-1000ppm. The methanol extract of Leptadenia hastata showed the high effect with activity indices of 0.73 ±0.23 at 25ppm and 1.16 ±0.06b 1000ppm but was highest with hexane extract 1.27±0.06b at 1000ppm against Staphylococcus aureus, relative to the control tetracycline, a standard drug (Table 2). This plant Leptadenia hastata has also been reported to play an important role in other human diseases, the plant is a good source of various secondary metabolites, which shows growth inhibition effect against bacteria, fungi, viruses, and tumours [24]. It is also used as laxative, antipyretic and expectorant, and in the treatment of infantile diarrhoea and malarial intermittent fevers [25]. Stomach problems, diarrhea, gonorrhea, malaria, cough, catarrhal conditions, diabetes, and as galactogogue [26]. In the management of onchocercosis, scabies, hypertension, catarrh, skin diseases, sexual potency, and wound-healing [27].
These studies is in agreement with the report of Alero and wara [28] that Methanol and water extracts from the leaves of Leptadenia hastata showed antibacterial activities. However, the activity observed from the hexane, dichloromethane, ethyl acetate and chloroform were all active in ascending order when compared with the drug control tetracycline. The action of Leptadenia hastata on Staphylococcus aureus is instructive.
The growth of Staphylococcus aureus was inhibited by these extracts at the tested concentration as shown in Table 2. The activity of this plant extracts against the Gram positive bacteria is quite responsive the methanol and hexane extract were more active than the chloroform, dichloromethane and ethyl acetate extracts with increase in concentration. This result is however suggesting the possibility for the treatment of diseases caused by this microorganism Staphylococcus aureus with increase in the concentrations. Antioxidant activity of the plant extracts was evaluated by DPPH radical scavenging mechanism. DPPH is a free radical compound that has widely been used to test the free radical scavenging abilities of various types of samples [29,30]. The antioxidant activities of leaf extracts of medicinal plants are given in Table 1. The results are shown as the relative activities against the standard ascorbic acid. The results showed that for n-hexane extracts tested it has the highest antioxidant activity of the leaf extract with DPPH inhibition of IC50 (63.44μg/mL).
The second most antioxidant activities against DPPH were displayed by the methanol extracts showing IC50 (46.80μg/ mL) which followed by chloroform, dichloromethane and lastly ethyl acetate crude extract. In comparison, the standard ascorbic acid showed IC50 (61.96μg/mL) DPPH inhibition in the assay. The screening and characterization of antioxidants derived from natural sources has gained much attention and efforts have been put into identifying compounds as suitable antioxidants to replace synthetic ones [31]. The presence of Leptadenia hastata leaf extract with potent antioxidant as shown in Table 1 continues to be of great importance in the search for remedies against free radical-mediated diseases, prevention of oxidative reactions in foods, protection against DNA damage, anti-bacterial, and anti-fungal properties
Conclusion
The result of this study showed that Leptadenia hastata (Pers) Decne extract contains bioactive components. Potentially, these compounds have the most important applications against human pathogens. The results of tests from various concentration and the presence of antioxidant component of the leaf extract indicate that the leafs have some measurable inhibitory action against grampositive bacteria such as Staphylococcus aureus.
Acknowledgment
The author acknowledged the contribution of colleague Thagriki Dluya and all supports from the Natural product laboratory FRST/ FSTS UNIMAS.
References
- Dewick PM (1996) Tumor inhibitor from plants in Trease and Evans Pharmacognosy. Elsevier Health Sciences Philadelphia, Pa, USA.
- Phillipson JD, Wright CW (1996) Plants with antiprotozoal activity in Trease and Evans Pharmacognosy, (14th edn), WB Saunders company, London, UK.
- Magherini R (1998) Le piante edicinalie aromaticheierie oggi Possibilita di coltivazione delte piante medicinalie aromaticheierie. Litalia Agricola vol. 3.
- Aliero BL, Umar MA, Suberu HA, Abubakar A (2001) A hand Book of common plant in northern Western, Nigeria, pp. 78.
- Tamboura HH, Bayala B, Lompo M, Guissoe IP, Sawadogo L (2006) Ecological Distribution, Morphological Characteristics and Acute Toxicity of Aqueous Extracts of Holarrhena floribunda (G. Don) Durand & Schinz, Leptadenia hastata (Pers.) Decne and Cassia sieberiana (DC) Used by Veterinary Healers in Burkina Faso. African Journal of Traditional, Complementary and Alternative medicines (AJTCAM) 2(1): 13-24.
- Betti JL, Yemefa’a SRM, Nchembi Tarla F (2011) Contribution to the knowledge of non-wood forest products of the far north region of Cameroon: Medicinal plants sold in the Kousséri market. J Ecol Nat Environ 3(7): 241-254.
- Ibrahim HA, Ali GY, Halliru SN, Usaini S, Abdullahi II (2012) Ethnobotanical Survey of the Wild Edible Food Plants Consumption among Local Communities in Kano State, North-Western, Nigeria. International Journal of Science and Technology 2: 713-717.
- Aquino R, Peluso G, De Tommasi N, De Simone F, Pizza C (1996) New polyoxypregnane ester derivatives from Leptadenia hastata. J Nat Prod 59(6): 555-564.
- Freiberger CE, Vanderjagt DJ, Pastuszyn A, Glew RS, Mounkaila G (1998) Nutrient content of the edible leaves of seven wild plants from Niger. Plant Foods Hum Nutr 53(1): 57-69.
- Nikiéma JB, Vanhaelen Fastré R, Vanhaelen M, Fontaine J, De Graef C (2001) Effects of anti-inflammatory triterpenes isolated from Leptadenia hastata latex on keratinocyte proliferation. Phytother Res 15(2): 131-134.
- Sena LP, Vanderjagt DJ, Rivera C, Tsin AT, Muhamadu I, et al. (1998) Analysis of nutritional components of eight famine foods of the Republic of Niger. Plant Foods Hum Nutr 52(1): 17-30.
- Dambatta SH, Aliyu BS (2011) A survey of major ethnomedicinal plants of Kano North Nigeria, their knowledge and uses by traditional healers. Bayero Journal of Pure and Applied Sciences 4(2): 28-34.
- Arbonnier M, Arbres (2000) Arbustes et Lianes des Zones Sèches d’Afrique de l’Ouest. (1st Edn.), CIRAD Publishers, Paris, p. 2-87614-431- X, 541.
- Wang H, Zhao M, Yang B, Jiang Y, Rao G (2008) Identification of polyphenols in tobacco leaf and their antioxidant and antimicrobial activities. Food Chemistry 107(4): 1399-1406.
- Tailor CS, Goyal A (2014) Antioxidant activity by DPPH radical scavenging method of Ageratum conyzoides Linn. leaves. American Journal of Ethnomedicine 1(4): 244-249.
- Ram Kumar P, Pranay J (2010) Comparative studies on the antimicrobial activity of black pepper (Piper nigrum) and turmeric (Curcuma longa) extracts. International Journal of Applied Biology and Pharmaceutical Technology 1(2): 491-501.
- Mahesh B, Satish S (2008) Antimicrobial activity of some important medicinal plant against plant and human pathogens. World journal of agricultural sciences 4(S): 839-843.
- Vandepitte J, Engback K, Piot P, Heuck CC (1995) Basic Microbiology Procedures in Clinical Bacteriology. Geneva: World Health Organization, pp. 85.
- Parekh J, Karathia N, Sumitra Chanda (2006) Screening of some traditionally used medicinal plants for potential antibacterial activity. Indian Journal of Pharmaceutical Sciences 68(6): 832-834.
- Maiyo ZC, Ngure RM, Matasyoh JC, Chepkorir R (2010) Phytochemical constituents and antimicrobial activity of leaf extracts of three Amaranthus plant species. African Journal of Biotechnology 9(21): 3178-3182.
- Eldeen IMS, Elgorashi EE, Van Staden J (2005) Antibacterial, antiinflammatory, anti-cholinesterase and mutagenic effects of extracts obtained from some trees used in South African traditional medicine. Journal of Ethnopharmacology 102(3): 457-464.
- Turnidge JD (2015) Susceptibility test methods: general considerations. In Manual of Clinical Microbiology (11th edn.), American Society of Microbiology, Washington, USA, pp. 1246-1252.
- Jorgensen JH, Turnidge JD (2015) Susceptibility test methods: dilution and disk diffusion methods. In Manual of Clinical Microbiology (11th edn), American Society of Microbiology, Washington, pp. 1253-1273.
- Ujwala K, Karpagam N (2013) Potential therapeutical values of plant latices. International Journal of Medicinal and Aromatic Plants 3(2): 317-325.
- Hoekou PY, Tchacondo T, Gbogbo KA, Tchelougou D, Pissang P, et al. (2015) Antibacterial activities of three latex plants of Asclepiadaceae family used in traditional medicine in South Togo. Int J Curr Microbiol App Sci 4(5): 882-891.
- Sutar NG, Pal SC (2014) Evaluation of analgesic activity of leaf extracts of Pergularia daemia[Forsk] in experimental animals. Int J Pharm Sci 6(9): 137-139.
- Thomas SD (2012) Leptadenia hastata: A Review of its Traditional uses and its Pharmacological Activity. Medicinal Chemistry 2(7): 144-150.
- Aliero AA, Wara SH (2009) Validating the medicinal potential of Leptadenia hastata. African Journal of Pharmacy and Pharmacology 3(6): 335-338.
- Sakanaka S, Tachibana Y, Okada Y (2005) Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food chemistry 89(4): 569-575.
- Naik GH, Priyadarsini KI, Satav JG, Banavalikar MM, Sohoni DP, et al. (2003) Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry 63(1): 97-104.
- Wong SP, LP Leong, JHW Koh (2006) Antioxidant activities of aqueous extracts of selected plants. Food Chem 99(4): 775-783.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...