Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4706
Research Article(ISSN: 2637-4706) 
Antimicrobial Activity of Selected Plant Extracts Volume 3 - Issue 4
Jagessar*
- Faculty of Natural Sciences, University of Guyana
Received: April 25, 2020; Published: May 11, 2020
Corresponding author: Jagessar, Department of Chemistry, Faculty of Natural Sciences, University of Guyana
DOI: 10.32474/DDIPIJ.2020.03.000167
Abstract
This paper survey the antimicrobial activity of selected plant extracts from the Guyanese flora. Those described here are the extracts of Artocarpusaltilis (breadfruit), Brassica rapaChinensis (pakchoi), Passifloraedulis (passion fruit) and leaves of Terminaliacatappa. These extracts were assayed via the disc diffusion assay at three different concentrations of 0.001g/ml, 0.05g/ml and 0.1g/ml. At 0.01g/ml, Brassica rapachinensis induces the higest AZOI of 106.9mm2 against S. aureus. A.altilis showed 0.0 AZOI against all pathogens. At 0.1g/ml, A. altilis showed highest AZOI of 89.2mm2. Zero AZOI was obtained against all pathogens, with exception S. aureus. The CH2Cl2 extract of A. altilis, showed the highest AZOI of 59.0mm2 against B.subtilis. For the ethanolic extract, Brassica rapachinensis at 0.01g/ml showed highest AZOI of 209.3mm2, whereas the ethanolic extract at 0.1g/ml showed the highest AZOI of 126.0mm2. Antimicrobial activity of the combined fruit extracts of Brassica rapachinensis and A. altilis revealed that at 0.01g/ml, there was an augmentation of antimicrobial activity for S. aureus (153.9mm2) and C. albicans (168.9mm2). For passion fruit, the highest AZOI of 153.9mm2 was observed against C. albicans (AZOI = 153.9mm2), whereas the lowest against K. pneumoniae (AZOI =15.9mm2). In all cases, antimicrobial potency was less than that of the reference compound. Also, small antimicrobial selectivity was observed. Antimicrobial activity of the aqueous extract of Terminaliacatappa, revealed that the highest AZOI of 254.71mm2 was induced against K. pneumoniae and least of 80.08mm2 against C. albicans. K. pneumoniae showing a selectivity factor of over C. albicans. The AZOI induced by the aqueous extracts were greater than the AZOI induced by reference compounds Ampicillin and Nystatin.
Keywords: Aqueous and Ethanolic extract; Passion fruit (Passifloraedulis); Asceptic conditions; Susceptible; Standard antibiotics; Selective antimicrobial activity; Transition metal salts; DZOI and AZO
Introduction
Research in the design and synthesis of antimicrobials will continue to be problematic on our planet, considering that bacteria and fungi develop resistance to antimicrobials over a period of time [1-5]. This results from indiscriminate use of commercial antimicrobial drugs for the treatment of infectious diseases and the current global antibiotic resistance [1-5]. Bacteria develop resistance to antibiotics via several means. These include the production of enzymes such as b-lactamase, which usually destroy the b-lactam ring of penicillin, thus rendering the drug inactive [6]. Figure 1 In another mode of antimicrobial resistance, transferases produced by enterococci can inactivate amikacin, gentamicin, and tobramycin (aminoglycosides) but not streptomycin.
Tetracyclines, also suffer antimicrobial resistance via [1] impaired influx or increased efflux by an active transport protein pump; [2] ribosome protection, due to production of proteins, that interfere with tetracylines, binding to the ribosome and [3]. enzymatic inactivation. Many synthetic drugs have several adverse side effects which are usually irreversible when administered and the cost of synthesizing drugs in most cases is an expensive endeavour [1-6]. Plants have a long therapeutic history over thousands of years and still considered to be promising source of medicine in the traditional health care system [7]. Plants also have a wide variety of secondary metabolites some of which are antimicrobial [8-10]. Crude plants extracts have also demonstrated antimicrobial activity [11-18].
Guyana flora is richly bio-diversified and its organic and aqueous extracts have been shown to possess potent and selective antimicrobial activity to date, compared with standard antibiotics: penicillin, nystatin and ampicillin [14-18]. In search of antimicrobials that have nutritional values (neutraceuticals), the use of the solventless extracts of leaves of Brassica rapachinensis, Artocarpusaltilis (Moraceae),Terminaliacatappa and passion fruit (passifloraedulis) against E. coli, S. aureus, K. pneumoniae, B. subtilis and C. albicansare discussed here.
Morphological Description and Scientific Classification of the Plant
Brassica rapachinensis (Brassicaceae) is a type of Chinese cabbage. They do not form heads and have green leaf blades with lighter bulbous bottoms, forming a cluster reminiscent of mustard greens. They are popularly grown in Guyana and flood markets, super makets. They are used as vegetables and are a rich source of vitamins (Figure 2).
Artocarpusaltilis (Moraceae) bread fruit tree usually grows to a height of 15m or more, with large incised leaves. The twigs are marked with the ring-scars of spathaceous stipules. The male flowers are borne in spike and female in spherical heads on the same plant [19]. The receptacle bearing the female flowers enlarges to a spherical stalked fruit, 20-30cm in diameter, at maturity [Figure 3]. The tree produces a fruit Called breadfruit.
Terminaliacatappa (Combretaceae) is a large tropical tree in the Leadwood tree family, Combretaceae. It grows to 35 metres (115ft) tall, with an upright, symmetrical crown and horizontal branches, [Figure 3]. It has corky, light fruit that is dispersed by water. The nut within the fruit is edible when fully ripe, tasting almost like almond. The leaves are large, 15-25cm long and 10-14cm broad, ovoid, glossy dark green and leathery. They are dry-season deciduous, before falling, they turn pinkish-reddish or yellow-brown, due to pigments such as violaxanthin, lutein, and zeaxanthin (Figure 4). The flowers are monoecious, with distinct male and female flowers on the same tree. Both are 1cm in diameter, white to greenish, inconspicuous with no petals. They are produced on axillary or terminal spikes [20,21]. Passion fruit, Passifloraedulis (Passifloraceae) is cultivated mostly in the tropics. The fruit is round to oval and usually yellow at maturity, with a soft to firm, juicy interior filled with numerous seeds, [Figure 5]. The fruit is both eaten and juiced or blended with other fruit juice to enhance aroma [19, 22].
It has several medicinal uses. These include: It boosts the immune system, it protects against cancer, heart diseases and premature ageing. It keeps skin hydrated and glowing. It improves eye health, aids in blood circulation in the body. Its beneficial in improving heart health, increases bone mineral density and bone strength. It also provides relief from constipation, facilitates healthy digestion of food and regulation of bowel movements. It reduces the risk of macular degeneration, cataracts and night blindness [22- 24].
Folklore and natural products constituents
Both Brassicarapachinensis, Artocarpusaltilis and their related species have medicinal uses and Natural Products/Phytochemicals with medicinal properties have been isolated from both plants and related species. Brassica rapachinensis and related species have antirheumatic, antiarthritic, antiscorbutic and resolvent properties [25]. Brassica rapa vegetables have been shown to possess glucosinolates with antioxidant properties [25,26]. The juice from the leaves of Brassica rapa species such as Turnip (Brassica rapa L.) have been shown to have hepatoprotective action through its antioxidative potentials [27].
Compounds isolated from related species of turnip (Brassica rapa ssp. campestris (Brassicaceae) have been shown to exhibit high inhibitory activity against the growth of human cancer lines, HCT-116, MCF-7, and HeLa, with IC50 values ranging from 15.0 to 35.0μM and against LDL-oxidation with IC50 values ranging from 2.9 to 7.1μM28. Phenolic natural products were isolated from Pak choi (Brassica rapachinensis) and seven other vegetables. These compounds were found to be hydroxybenzoic acids, hydroxycinnamic acids and flavonoids. Salicylic acid was found to be the most common hydroxybenzoic acid, ranging from 4.40 to 117.36μg/g fresh frozen weight (ffw). Vanilic, gallic, caffeic, chlorogenic, p-coumaric, ferulic and m-coumaric acids were also found in all of these vegetables. Isoquercetin and Rutin, the most common flavonoids, ranged from 3.70 to 19.26 and 1.60 to 7.89μg/gffw, respectively [28-31].
Phytochemical, and spectroscopic investigations of related species of turnip (Brassica rapa ssp. campestris (Brassicaceae) revealed the presence of a novel phenanthrene derivative, 6-methoxy-1-(10-methoxy-7-(3-methylbut-2-enyl) (phenanthren-3-yl) undecane-2,4-dione, brassica phenanthrene, along with two known diarylheptanoid compounds, 6-paradol and trans-6-shogaol. These compounds have been reported to have anticancer activity [32].
The breadfruit (Artocarpusaltilis) is edible. The leaves and the sap have been used for various medicinal purposes. The tea of breadfruit leaves is used to lower blood pressure and treat diabetes. The sap is applied to contagious skin ailments to prevent their spreading and promote healing [19]. Artocarpusaltilis leaf extracts have been shown to have cytoprotective, anti-inflammatory (leaves), cytotoxic, negative inotropic effect, anti-cancer, antitubercular and anti-plasmodial activities. An ethanol extract of the leaves showed potent ACE (Angiotensin-converting enzyme) inhibitory activity, supporting it’s use in folk medicine for the treatment of hypertension. The isolated compounds exhibited antitubercular and anti-plasmodial activities [33-35].
Artocarpusaltilis leaf extracts were investigated against angiotensin-converting enzyme (ACE) activity. Amongst the extracts tested, hot ethanol extract exhibited a potent ACE-inhibitory activity with an IC₅₀ value of 54.080.29µgmL⁻¹, followed by cold EtOAc extract (IC₅₀ of 85.44±0.85µgmL⁻¹). In contrast, the hot aqueous extracts showed minimum inhibition with the IC₅₀ value of 765.5211.97µgmL⁻¹ at the maximum concentration tested. The high content of glycosidic and phenolic compounds could be involved in exerting ACE-inhibitory activity, supporting the utilisation of A. altilis leaf in the folk medicine for the better treatment of hypertension [36-37].
Phytochemical and spectroscopic studies of the methanol extract of Artocarpusaltilis, resulted in the isolation and spectroscopic characterization of a new prenylatedaurone, artocarpaurone, together with eight known compounds, including two prenylatedchalcones, three prenylated flavanones, and three triterpenes. The structure of the new compound was elucidated as 6-hydroxy-2-[8-hydroxy-2-methyl-2-(4-methyl-3-pentenyl)-2H-1-benzopyran-5-ylmethylene)-3(2H)-benzofuranone. It showed moderate nitric oxide radical scavenging activity, whereas two compounds had moderate 2,2-diphenyl-1-picrylhydrazyl radical scavenging effect, compared with the positive control (+)-catechin [38].
Anti-tubercular and anti-malarial activity-guided study of the roots of Artocarpusaltilisled to the isolation of nine prenylated flavones, Figure 6. Cycloartocarpin (1), Artocarpin (2), and Chaplashin (3) were isolated from the CH2Cl2 extract of the root stems, whereas Morusin (4), Cudraflavone (5), Cycloartobiloxanthone (6), Artonin(7), Cudraflavone(8) and Artobiloxanthone (9) were found in the root barks. The isolated compounds exhibited antitubercular and antiplasmodial activities, and also showed moderate cytotoxicity against KB (human oral epidermoid carcinoma) and BC (human breast cancer) cell lines [39].
The cytoprotective effects of various solvent extracts of Artocarpusaltilis (Parkinson) Fosberg were evaluated. These effects were determined in human U937 cells incubated with oxidized LDL (OxLDL) using the 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)- 2H-5-tetrazolio]-1, 3-benzenedisulfonate (WST-1) assay. Results demonstrated that the EtOAc extract showed cytoprotective activities. To identify the main cytoprotective components, a bioassay guided isolation of the ethyl acetate extract afforded b-sitosterol and six flavonoids. Their chemical structures were established on the basis of spectroscopic evidence and comparison with literature data. One of these compounds was obtained from A. altilis for the first time. The cytoprotective effect offers good prospects for the medicinal applications of A. Altilis [40].
Flavonoids 10-oxoartogomezianone, 8-geranyl-3-(hydroxyprenyl) isoetin, hydroxyartoflavone, isocycloartobiloxanthone, and furanocyclocommunin, together with 12 known compounds, were isolated from heartwood and cortex of Artocarpusaltilis, and were spectroscopically characterized. The flavonoids isolated from A. altilis may be suspected candidate antioxidants and/or skin-whitening agents [40].
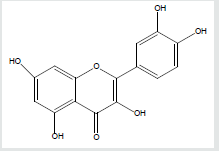
Leaves of Terminaliacatappa contain several flavonoids such as kaempferol or quercetin), Figure 7 Terminaliacatappa contains hydrolysed tannins such as punicalagan as a major component, punicalin, terflavins A and B, tergallagin, tercatain, chebulagic acid, geranin, granato B, corilagin, flavanoids (isovitexin, vitexin, rutin, triterpenoids (ursolic acid, 2, 3, 23-trihydroxyurs, 12-en-28 oic acid, Asiatic acid), squalene but no caffeine [41-45]. The leaves and also the bark are used in different traditional medicines for various purposes. In Taiwan, fallen leaves are used as an herb to treat liver diseases. In Suriname, a tea made from the leaves is prescribed against dysentery and diarrhea. The leaves are thought to contain agents for prevention of cancers, although they have not demonstrated anti-carcinogenic properties and antioxidant as well as anti-clastogenic characteristics. The leaves kept in an aquarium is said to lower the pH and heavy metal content of the water. It's also believed that it helps prevent fungus forming on the eggs of the fish [46]. The essential oil from the leaves of Terminaliacatappa L. was analyzed by gas chromatography and mass spectrometry (GC-MS). The leaf oil was dominated by (Z)-phytol (41.2%), palmitic acid (11.0%), and (E)-nerolidol (4.7%). Alkane hydrocarbons (25.5%) made up a significant portion of the leaf oil composition [42].
A study was done to quantify the majority compounds of the hydroalcoholic extract (7: 3, v/v) of the leaves from T. catappaby HPLC-PDA, chemically characterize by hyphenated techniques (HPLC-ESI-IT-MSn) and NMR. The quantification of analytes was performed using an external calibration standard. Punicalagin is the most abundant polyphenol found in the leaves [43]. The presence of this compound as a mixture of anomers was confirmed using HPLC-PDA and 1H and 13C NMR [43].
Passion fruit is rich in polyphenols [22,44]. The fruit also contain prunasin and other cyanogenic glycosides in the peel and juice [45]. Passion fruit oil is composed mainly of linoleic acid (77%) with smaller amounts of oleic acid (15%) and palmitic acid (10%). It also contains vitamin C (36%), dietary fiber (42%), B vitamins riboflavin (11%) niacin (10%), iron (12%) and phosphorus (10%) in significant percentages of the daily value [45]. The structure of some of these natural products are shown in Figure8
Literature Review
Antimicrobial activity of Brassica rapa L (turnip) roots extracts (light petroleum ether, chloroform and ethylacetate and aqueous) was investigated against C. albicans, P. aeruginosa and B. subtilis. Light petroleum fraction of roots showed somewhat strong antifungal activity against Candida albicans with MIC calculated as 12.5mg/ml [46].
In vitro antimicrobial activity of Artocarpusaltilis was evaluated against six pathogenic microorganisms (two gram positive strains: Staphylococcus aureus, Bacillus subtilis, the Gram negative strains Escherichia coli and Pseudomonas aeruginosa and two fungal strains: Candida albicans and Cryptococcus neoformans, using the disc diffusion method at 2mg/disc, by indicating the presence of the clear inhibition zones around each disc, compared with the positive control (Streptomycin and Nystatin), while the MIC and MBC/MF ranged from (250-1000µg/ml). The extracts of hexane and DCM induced a moderate antimicrobial activity at (14.6±0.2mm) against Bacillus cereus, whereas 11.8±0.3mm and 11.5±0.2mm values for C. albicans, respectively. The least MIC of 250µg/ml was obtained by DCM extract against S. aureus for bacteria, C. albicans and C. neoformans for fungus, respectively. The results suggested that Artocarpusaltilis extracts have promising therapeutic potential against bacteria and fungi [47,48].
A study was conducted to determine whether the methanol extract of breadfruit leaves variations (Artocarpusaltilis) have activity on Escherichia coli, Staphylococcus epidermidis, Propionibacterium acnes and Candida albicans and determination of Minimum Inhibitory Concentration (MIC). The test results showed antibacterial and antifungal activity variations of fermented green gives effective results than other breadfruit leaf variations. MIC values for the bacterium Escherichia coli, Staphylococcus epidermidis, Propionibacterium acnes, and the Fungi Candida albicans in a row by fermented green extract 20mg/mL; 47, 5mg/mL; 15mg/mL; and 475mg/mL.
The extract of P. edulis Sims var. edulis seeds showed good antimicrobial effects on P. acnes and the inhibitory activity increased with concentration of the extract. Comparable inhibitory effects with clindamycin and erythromycin may support the application of this extract in the management of acne vulgaris. Further clinical trials on the use of P. edulis Sims var. edulis seeds extract are necessary to show its efficacy in acne vulgaris patients [49].
The methanol, acetone and N,N-dimethylformamide extracts of Terminaliacatappa I. leaves were evaluated for antibacterial and antifungal activity using the agar disc diffusion method. Piperacillin and gentamicin were used as standards for antibacterial assay, while nystatin and fluconazole were used as standards for antifungal assay. The antibacterial activity was more pronounced against bacteria than fungal strains. The Gram positive bacteria were more susceptible than Gram negative bacteria. The best antibacterial activity was noted for the methanol extract. In addition, Terminaliacatappa leaf extracts showed better antibacterial activity than Piperacillin and gentamicin [50].
The effect against bacteria of petroleum ether (60-80, °C), chloroform and methanolic extract of dried root of Terminaliacatappa Linn. (combretaceae) was employed by cup plate agar diffusion method. The chloroform extract showed prominent antimicrobial activity against S. aureus and E. coli, as compared to other tested microorganisms, while petroleum ether extract was devoid of antimicrobial activity. The methanolic: extract exhibited MIC of 0.065mg/ml against E. coli. and chloroform extract exhibited MIC of 0.4mg/ml against S. aureus. The chloroform as well as methanolic extracts showed good antimicrobial activity against Gram positive and Gram-negative microorganisms [51].
Aqueous and methanol extract of the leaves of Terminaliacatappa L., Manilkarazapota L. and Piper betel L. were evaluated for their antibacterial activity against ten (10) Gram positive, twelve (12) Gram negative bacteria and one fungal strain, Candida tropicalis. Piperacillin and gentamicin were used as standards for antibacterial assay, while fluconazole was used as standard for antifungal assay. The three plants showed different degree of antimicrobial activity against the microorganisms investigated. The methanolic extract was considerably more effective than aqueous extract in inhibiting the investigated microbial strains. The most active antimicrobial plant was Piper betel [52].
Methodology
The extracts of the above-mentioned plant parts were investigated for their antimicrobial activity against pathogens [52] using the disc diffusion approach. The description of these methods can also be found in standard Microbiological texts.
Collection of plant materials
Breadfruit and Pak choi leaves were collected from a local farm, whereas passion fruits in a semi-ripe state were purchased from a vendor at the Bourda market. Terminaliacatappa leaves were collected from the University of Guyana environment. Each fruit was washed, rinsed with distilled water and then subjected to aerial drying and were then weighed. The same was noted for Terminaliacatappa leaves. Both dried breadfruit and Pak Choi leaves were severed into small pieces, whereas each passion fruit was sliced in two halves. Terminaliacatappa leaves were ground to a fine material using a grinding mill. Each plant parts was then placed in extraction jars and extracted sequentially with solvents of varying polarity: n-C6H14, CH2Cl2 and CH3CH2OH. n-C6H14, CH2Cl2 and CH3CH2OH solvents were freshly distilled before use. After extraction, solvents were filtered and dried over Na2SO4. Solvents were removed in vacuo using the rotatory evaporator, resulting in viscous oils, solids and pastes. Figure 9 shows a typical solvent extraction of plant material.
Preparation of extract solution for antimicrobial studies
A specified amount of dried crude extract of the plant material (fruit and leaves)was weighed and transferred to a 10 ml volumetric flask, such that the concentration was 0.01g/ml and 0.1 g/ml for n-hexane, dichloromethane, ethanol and aqueous.
Aseptic conditions
Antimicrobial assay was done under asceptic conditions. The aseptic chamber which consists of a wooden box (1m x 1m x 0.5m) with a door, was cleaned with 70% ethanol and irradiated with short wave UV light (from a lamp). A sterilized 6mm cork borer was used to cut agar discs in the plate for the Well-Diffusion method. All agar plates, sample vials etc were sterilized in an autoclave, prior to use.
Source of microorganisms
Pathogenic microorganisms: Staphylococcus aureus, Escherichia coli, Klebsielapneumoniae, Bacillus subtilis and Candida albicans were obtained from the Georgetown Public Hospital (GPHC) microbiology laboratory and were stored in a refrigerator at the University prior to the experiment.
Bacteria used were: Staphylococcus aureus (ATCC 25923) Escherichia coli and Candidaalbicans (ATCC 1023) in nature. 3-5 colonies from an overnight plate was transferred to a tube containing 10ml of distilled water and mixed. The solution was compared with the 1.0 McFarland Standard and the density was adjusted either by adding more colonies or adding more distilled water to the tube. The choice of the agar used for bacterial and fungal growth was nutrient agar which accommodates non fastiduos growth of microbes. All microorganisms obtained were cultured in a Luria-Bertani broth.
Luria-Bertani broth (LB broth) is a rich medium used to culture bacteria such as E. Coli and S. aureus. To make it, tryptone (10g), yeast extract (5g) and sodium chloride (10g) were weighed and placed in a 1L cylinder. Distilled water was added to make up the 1L solution and the mixture was poured and re-poured until the contents were dissolved. The pH of the solution was adjusted to 7.4 using NaOH. 3mL each of LB broth was placed in 56 test tubes. The tubes were plugged with cotton wool foil and wrapped over each top. The tubes were placed into a beaker and autoclaved at 121 °C for 2h. These tubes were used in the dilutions experiments.
Turbidity (opacity) standard
This is the barium chloride standard against which the turbidity of the test and control innocula can be compared. When matched with the standard, the inocula should give semi-confluent growth. The turbidity of the standard is equivalent to an overnight broth culture.
Preparation of turbidity standard (0.5 McFarland)
1% v/v sulphuric acid: 1ml of concentrated H2SO4 was added to 99ml of distilled water. 1.175% w/v of barium chloride solution: 2.35g of barium chloride, BaCl2.2H2O was dissolved in 200ml of distilled water. To make the turbidity standard, 0.5ml of the barium chloride solution was added to 99.5ml of the sulphuric acid solution and thoroughly mixed. The standard solution was dispersed into screw cap tubes as the same type as those for the preparation of the test and control innocula. It was stored in the dark at room temperature.
Microbial medium: Two types of agar were used, nutrient
agar to make up the medium for bacteria and PDA (Potato Dextrose
Agar) to make up the medium for fungi [38-41]. The potato was
peeled and 100g was measured, finely chopped and boiled to a
mash in distilled water. Dextrose was weighed (12.5g) and placed
in a 1L measuring cylinder. Agar was measured (12.5g) and added
to the measuring cylinder (with the dextrose). The potato mash
was stirred and strained into the cylinder. Hot distilled water was
added to make up to 500mL. The contents was continuously poured
and stirred until consistency was achieved. The content was then
poured into a conical flask, plugged with cotton wool, over which
aluminium foil was tightly wrapped. The flask was then autoclaved
at 121 0C for 24hrs. The pH range was between 6.5-7.0.
For the preparation of the nutrient agar medium, 20g of nutrient
agar was suspended in 500ml of distilled water in a 1L flask and
stirred. The pH range was between 7.0-8.0. The conical flask was
then plugged with cotton wool and wrapped in aluminum foil, then
autoclaved at 121°C for 15 minutes. The sterilized medium was
then poured into the sterilized 90mm sterilised petri-plates and
allowed to cool to a depth of 4mm and solidify for two hours. These
plates were allowed to cool and refrigerated for use the following
day.
Ant microbiological susceptibility tests
Plant extracts were investigated for their antimicrobial activity using the Agar Disc diffusion 59-61 techniques under aseptic conditions or Stokes Disc diffusion sensitivity techniques. Using Stokes Disc diffusion sensitivity testing technique [38-41], an inoculum containing bacterial or yeast cells was applied onto nutrient agar plates, prepared and stored overnight. On each plate, a reference antibiotic was also applied. The reference antibiotic disc contained 10mg of antibiotic/disc. The discs were made by cutting discs (5-6mm) from a filter paper with a perforator, placing 5 of these discs in a vial and adding 0.2mL of each extract solution. These were left to dry. Discs were also made for the controls: ampicillin for the bacteria and nystatin for the fungus. Each disc was impregnated with the anticipated antimicrobial plant extract at appropriate concentration of 200mg/ml using a microlitre syringe.
This was then placed on a plate of sensitivity testing nutrient agar which was then incubated with the test organism: Bacteria/fungi. Incubation was done at 37 ºC for 24hr and 48hr for the bacteria and Candida albicans species respectively. The antimicrobial extract diffuses from the disc into the medium. Following overnight incubation, the culture was examined for areas of no growth around the disc (zone of inhibition). The radius of the inhibition zone was measured from the edge of the disc to the edge of the zone. The end point of inhibition is where growth starts. Larger the inhibition zone diameter, greater is the antimicrobial activities. It is anticipated through the antimicrobial activity of plant extract, no area of growth will be induced around the disc. Bacteria or fungal strains sensitive to the antimicrobial are inhibited at a distance from the disc whereas resistant strains grow up to the edge of the disc. Discs applied to the plates already streaked with bacteria and the fungus. It is anticipated through the antimicrobial activity of plant extract, no area of growth will be induced around the disc. Interpretation of susceptibility are made by comparing the sizes of zones of inhibition to a standard reference table.
Reference and control
For both methods, the control experiment consists of solidified agar onto which was applied solvents: n-hexane, CH2Cl2, ethanol and EtOAc. The reference experiment (positive control) consist of the application of Ampicillin disc or Nystatin disc on agar plates inoculated with microbial strains E. coli, S. aureus, K. pneumoniae and C. albicans respectively. Ampicillin was used specifically for all bacteria Staphylococcus aureus and Escheria coli, whereas Nystatin was used against fungal strains such as Candida albicans. The Control experiment consists of a plate of solidifying agar onto which was inoculated pure solvent with microorganism mixed in a 1:1 portion [38-41].
Thin layer chromatography (TLC)
A baseline was drawn on the TLC plate. A spot of the plant extract
was placed on the baseline with use of the pipette and allowed to
dry. The plate was placed in the developing jar with the solvent.
When taken out of the jar, the solvent front was drawn. The plates
were then held in the iodine jar for a few seconds, shaken and taken
out. They were examined under the UV/Vis lamp and the spots
were circled with a pencil. The plate was further examined under
UV lamp and any new spots were marked. The spots were labelled
and their distances from the baseline were measured. The distance
between the baseline and the solvent front was also measured. The
Rf values were calculated in Figure 10 & 11, Graphs 1-5.
Figure 10: The effect of the administration of aqueous extract of Terminaliacatappaagainst (a)Staphylococcus aureus and (b) Klebsiellapneumoniaemicrobial strains.

Graph 1: A Plot of AZOI induced by hexane extract of leaves of B. chinensis and A. altilis at 0.01g/ml.

Graph 2: A Plot of AZOI induced by hexane extract of leaves of B. chinensis and A. altilis against selected pathogens at 0.1g/ ml.

Graph 3: A Plot of AZOI induced by combined fruit extracts of leaves of Brassica Chinensis and Artocarpusaltilis at concentration of 0.01g/ml and 0.1g/ml.

Graph 4: A Plot of AZOI induced by ethanolic extract of passion fruit, Passifloraedulis at 0.01g/ml against selected pathogens.

Graph 5: A Plot of AZOI induced by aqueous extract of leaves of Terminaliacatappa against selected pathogens.

Results
Table 1 Antimicrobial activity of n-C6H14 extract of leaves of Brassica rapachinensis and A. altilisat 0.01g/ml and 0.1g/ml concentration.
Discussion
Results are shown in Table 1 to Table 11.
Table 1 shows the antimicrobial activity of the n-C6H14 extracts of leaves breadfruit and vegetable, pakchoi, Brassica rapachinensis at 0.01g/ml and 0.1g/ml concentration, against the various pathogens. At 0.01g/ml, Artocarpusaltilis wasn’t antimicrobial (zero AZOI), whereas Brassica rapachinensis was antimicrobial, with AZOI, ranging from 34.9mm2 to 119.3mm2. As the hexane extract concentration was increased to 0.1g/ml, Artocarpusaltilis still display zero antimicrobial activity, with the exception against Bacilussubtilis, an AZOI of 89.2mm2 was induced. Thus, at this concentration, the hexane extract display antimicrobial selectivity. For Brassica rapachinensis, non-antimicrobial activity was also induced against the pathogens, with an exception against S. aureus (AZOI = 38.5mm2) and C. albicans (AZOI =73.4mm2).
Table 1:Antimicrobial activity of n-C6H14 extract of leaves of Brassica rapachinensis and A. altilisat 0.01g/ml and 0.1g/ml concentration.

Only the antimicrobial activity of CH2Cl2 extract of A. altilis was investigated at the three concentrations of 0.01g/ml, 0.05g/ml. and 0.1g/ml, Table 2. CH2Cl2 extract of A. atilis at 0.01g/ml and 0.05g/ml showed antimicrobial selectivity against B. subtilis, with AZOI of 59.0mm2 and 46.2mm2, respectively. A. altilis extract at 0.1g/ml, showed antimicrobial selectivity against S. aureus (AZOI = 54.1mm2). At all other three concentrations, zero AZOI was induced against all the other pathogens.
Table 2: Antimicrobial activity of CH2Cl2 extract of leaves of A. altilisat 0.01g/ml, 0.05g/ml and 0.1g/ml concentration.

Table 3 illustrates the antimicrobial activity of CH3CH2OH extract of leaves of Brassica rapachinensis and A. altilis at 0.01g/ml and 0.1g/ml concentration. At 0.01g/ml, the highest AZOI of 209.3mm2 was induced by leaves of Brassica rapachinensis against E.coli and the lowest against B. subtilis (12.6mm2). The corresponding AZOI against A. altilis range from 0.0 to 94.9mm2, with the highest of 94.9mm2, induced against S. aureus. When the ethanolic extract concentration was increased to 0.1g/ml, it was noticeable that the highest AZOI of 126mm2 was induced against C. albicans. Comparatively, Artocarpusaltilis extract induced 0.0mm2 AZOI against E. coli, S. aureus and B. subtilis. Only an AZOI of 59.00mm2 was induced against C. albicans.
Table 3: Antimicrobial activity of CH3CH2OH extract of leaves of Brassica rapachinensis and leaves of A. altilisat 0.01g/ml and 0.1g/ ml concentration.

Table 4 shows the antimicrobial activity of combined ethanolic extracts of Brassica rapachinensis and A. altilis at a concentration of 0.01g/ml. For the 0.01g/ml concentration, the highest AZOI of 168.9mm2 was induced against C. albicans and the lowest against B. subtilis (67.8mm2). The combined hexane extract of A. altilis and Brassica rapachinensis at 0.01g/ml, showed an AZOI of 73.4mm2 against B. substilis and zero AZOI against E. coli, S. aureus and C. albicans. Hence, antimicrobial selectivity against B. subtilis, in comparison to E. coli, S. aureus and C. albicans.
Table 4: Antimicrobial Proficiency of Combined ethanolic and hexane fruit extracts of leaves of A. altilis and Brassica rapachinensis.

Table 5 shows the antimicrobial activity of passion fruits against selected pathogens: E. coli, K. pneumoniae, S. aureus, P.aeruginosa and C. albicans at three different concentrations. The highest AZOI of 153.9mm2 was induced against C. albicans at a concentration. of 0.01g/ml. The lowest AZOI of 15.9mm2 was induced against K. pneumoniae at a concentration of 0.1g/ml.
In comparison to reference compound, the highest AZOI of 153.9mm2 induced against C. albicans was approximately 30%, [Table 6]. Increasing the ethanolic passion fruit concentration, resulted in an increase in antimicrobial potency in all cases, with the exception against C. albicans. Exception being a decrease in antimicrobial potency at 0.1g/ml concentration against K. pneumoniae and C. albicansat 0.01g/ml concentration.
Table 6: A comparison of the antimicrobial potency of the fruit extract (Passifloraedulis) versus that of standard antibiotics.

Table 7 shows the AZOI induced by the aqueous extract of Terminaliacatappa. The largest AZOI of 254.71mm2 was induced against K. pneumoniae, whereas the lowest AZOI of 80.08mm2 was induced against C. albicans. The AZOI range from 80.08mm2 to 254.71mm2. Compared to the Reference compound, Ampicillin and Nystatin, these AZOI were significantly higher Figure 8. For example, against K. Pneumoniae, aqueous extract of Terminaliacatappa, induced AZOI of 254.71mm2 against K. pneumoniae, whereas the reference compound, Ampicillin induced a corresponding AZOI of 44.2mm2. Figure 9 shows the effect of the administration of ethanolic extract of passion fruit against S. aureus at increasing concentration. Figure 10 shows the effect of the administration of aqueous extract of Terminaliacatappa against selected pathogens.
TLC (Thinlayer chromatography of Brassica rapachinensis and Artocarpusaltilis hexane extracts revealed the presence of four and three spots respectively, whereas TLC analyses of the ethanolic extract, revealed the presence of two spots respectively. Each spot is presumably due to a pure natural product. Statistically, Two-Factor ANOVA, with Replication can be used to analyse whether significant differences exist in the diameter of zone of inhibition between concentration of extracts and organisms62-63. As an example, consider, passion fruit, Table 8 shows that differences between samples were significant throughout since the calculated p values is greater than 0.05 and that F value is greater than F critical. Table 9 shows that differences between samples were not significant throughout, since the calculated p-values is less than 0.05 (Table 10 & 11).
Table 7: AZOI induced by the aqueous extract of Terminaliacatappa (TC) at 0.01g/ml on selected pathogens.

Table 8:Area of Zone of Inhibition, induced by the reference compound, Ampicillin and Nystatin against the selected pathogens in comparison to Terminaliacatappa extract.

Table 9: Comparison of zones of inhibition between organisms and samples using ANOVA Two Factor without Replication (p < 0.05 = insignificant).

Table 10:Shows comparison of zones of inhibition when different extracts are applied to microbial cultures using ANOVA single factor (p < 0.05 = insignificant).

Table 11: TLC Profile of CH3CH2OH and C6H12 extract of Brassica rapa Chinensis and Altocarpusaltilis extract.

Conclusion
Antimicrobial activity of leaves of Brassica rapachinensis, Artocarpusaltilis, Passifloraedulis and Terminaliacatappa, were investigated via the disc diffusion assay, where the diameter of zone of inhibition (DZOI) and the corresponding AZOI were used as indicator of plant extracts antimicrobial potency. The hexane and ethanolic extract of Brassica rapachinensis, Artocarpusaltilis and the ethanolic and aqueous extracts of passion fruit, Passifloraedulis and Terminaliacatappa were investigated respectively. Comparing the ethanolic extract, the highest AZOI of 209.3mm2 was induced by the leaves extract of Brassica rapachinensis against E. coli. The lowest of zero, was induced by the ethanolic extract of Artocarpusaltilis against all pathogens. Both the ethanolic and aqueous extract of passion fruit, passifloraedulis and Terminaliacatappa were antimicrobial against all pathogens. For the former, the AZOI ranges from 32.2mm2 to 153.9mm2. For the latter, AZOI, ranges from 80.08mm2 to 254.71mm2. Antimicrobial selectivity was also observed. Only Terminaliacatappa aqueous extract was found to be more antimicrobial than the reference compounds, Ampicillin and Nystatin. Thus, all plants species investigated can be utilized for their antimicrobial activity in addition to their nutritional value. Figure 11. The fact that the antimicrobial activity of Terminaliacatappa is greater than standard antibiotics warrants its use as a potential topical bactericidal agent.
References
- Wilms LR (2009) Guide to Drugs in Canada. Leo Paper Products Ltd. Third Edition 114-117.
- Macor JE (2010) Annual reports in Medicinal Chemistry. the American Chemical Society 45: 295-311.
- Wood A (2008) Topics in Drug design and discovery. Annual Reports in Medicinal Chemistry 41: 353-409.
- Bonner J (2009) Filling the Antibiotic Gap. Chemistry World, Royal Society of Chemistry 6(8): 16.
- Kelland K (2013) Antibiotic Resistance Poses Catastrophic Threat to Medicine”. Huffington Post 1-3.
- Smith, CM Reynard AM (2012) Textbook of Pharmacology. WB Saunders company, (3rd), 96-1174.
- Shahid W, Durrani R, Iram S, Durrani M, Khan F (2013) Antibacterial activity in Vitro of medicinal plants. Sky Journal of Microbiology Research 1(2): 5-21.
- Rijo P, Faustino C, Simoes MF (2013) Antimicrobial natural products from Plectranthus plants Microbial pathogens and strategies for combating them: science, technology and education. 922-931.
- Arif T, Bhosale JD, Kumar N, Mandal TK, Bendre RS, et al. (2009) Natural Products-antifungal agents derived from plants. J Asian NatProd Res 11(7): 621-638.
- Melgarejo M, Mollinedo P, Castro JV (2006) Antibacterial activity of four Natural Products from a Bolivian Highland Plant Revista Boliviana De Quimica 23(1): 40-43.
- Saswati R Choudhury M, Paul S (2013) In Vitro Antibacterial Activity of Alocasia Decipiens Schott, International Journal of Pharmacy and Pharmaceutical Sciences 5.
- Aarati N, Ranganath N, Soumya G, Kishore B, Mithun K (2011) Evaluation of Antibacterial and Anticandidal Efficiency of Aqueous and Alcoholic Extract of Neem, Azadirachtaindica International Journal of Research and Pharmaceutical.
- Meenal K, Khadabadi S, Saboo S, Ghorpade D, Modi A (2010) In Vitro Antimicrobial Activity of The Crude Extracts of Colocasia Esculenta Leaves (Araceae). International Journal of Pharmacy and Pharmaceutical Sciences 1: (8).
- Jagessar RC, Mohamed N (2010) Antimicrobial activity of selected plants extracts from Guyana’s flora”. Journal of Pure and Applied Microbiology 4(2): 533-540.
- Jagessar RC, Allen R (2011) Antimicrobial Potency of the Aqueous Extract of leaves of Terminaliacatappa. Academic Research International 362-371.
- Jagessar RC, Mars A, Gomathigayam S (2011) Selective Antimicrobial properties of Leaf extract of SamaneaSaman against Candida albicans, Staphylococcus aureus and Escherichia coli using several microbial techniques. Journal of American Science 7(3): 108-119.
- Jagessar RC, Mars A, Gomes G (2009) Leaf extract of Smilax Schomburg Kiana exhibit selective antimicrobial properties against pathogenic microorganisms. Life Science Journal 6(1): 76-83.
- Jagessar RC, Mohammed A, Gomes G (2008) An evaluation of the antibacterial and antifungal activity of leaf extracts of Momordica Charantia against Candida albicans, Staphylococcus aureus and Eschericia Coli Nature and Science 6(1): 1-14.
- Pankaj Oudhia, Robert E Paull (2008) West Indian Almond Terminaliacatappa L. Combretaceae. Encyclopedia of Fruit and Nuts. 273-276.
- Talcott ST, Percival SS, Pittet-Moore J, Celoria C (2003) Phytochemical composition and antioxidant stability of fortified yellow passion fruit (Passifloraedulis). J Agric Food Chem 51(4): 935-941.
- Devi Ramaiya S, Bujang JS, Zakaria MH, King WS, Shaffiq Sahrir MA (2013) Sugars, ascorbic acid, total phenolic content and total antioxidant activity in passion fruit (Passiflora) cultivars. J Sci Food Agric 93(5): 1198-1205.
- Chassagne D, Crouzet JC, Bayonove CL, Baumes RL (1996) Identification and Quantification of Passion Fruit Cyanogenic Glycosides. J Agric Food Chem44 (12): 3817–3820.
- Duke JA, Ayensu ES (1985) Medicinal Plants of China. Inc. Algonac Michigan, China.
- Vicas SI, Teusdea AC, Carbunar M, Socaci SA, Socaciu C (2013) Glucosinolates Profile and Antioxidant Capacity of Romanian Brassica Vegetables Obtained by Organic and Conventional Agricultural Practices. Plant Foods for Hum Nutr 68(3): 313-321.
- Rafatullah S, Al-Yahya Ml, Mossa J, Galal A, El-Tahir K, (2006) Preliminary Phytochemical and Hepatoprotective Studies on Turnip Brassica rapa L International Journal of Pharmacology 2(6): 670-673.
- Wu Q, Cho JG, Yoo KH, Jeong TS, Park JH, et al. (2013) A new phenanthrene derivative and two diarylheptanoids from the roots of Brassica rapa ssp. campestris inhibit the growth of cancer cell lines and LDL-oxidation. Arch of Pharm Res 36(4): 423-429.
- Vicas SI, Teusdea AC, Carbunar M, Socaci SA, Socaciu C (2013) Glucosinolates Profile and Antioxidant Capacity of Romanian Brassica Vegetables Obtained by Organic and Conventional Agricultural Practices. Plant Foods for Hum Nutr 68(3): 313-321.
- Rafatullah S, Al-Yahya M l, Mossa J, Galal A, El-Tahir K (2006) Preliminary Phytochemical and Hepatoprotective Studies on Turnip Brassica rapa L. International Journal of Pharmacology 2(6): 670-673.
- Siddesha J M, Angaswamy N, Vishwanath BS (2011) Phytochemical screening and evaluation of in vitro angiotensin-converting enzyme inhibitory activity of Artocarpusaltilis Nat Prod Res 25(20): 1931-1940.
- Khair U, Khanama S, Obab S, Yanaseb E, Murakamic Y (2012) Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. Journal of Functional Foods 4(4): 979-987.
- Huong TT, Cuong NX, Tram l H, Quang TT, Duong lV, et al. (2012) A new prenylatedaurone from Artocarpusaltilis. J Asian Nat Prod Res 14(9): 923-928.
- Khair U, Khanama S, Obab S, Yanaseb E, Murakamic Y (2012) Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. Journal of Functional Foods 4(4): 979-987.
- Boonphong S, Baramee A, Kittakoop P, Puangsombat P, (2007) Antitubercular and Antiplasmodial Prenylated Flavones from the Roots of Artocarpusaltilis, Chiang Mai. Journal of Science, 34(3): 339-344.
- Wang Y, Deng T, Lin L, Pan Y, Zheng X (2006) Bioassay‐guided isolation of antiatherosclerotic phytochemicals from Artocarpusaltilis. Phytother Res 20(12): 1052-1055.
- Lan WC, Tzeng CW, Lin CC, Yen FL, Ko HH (2013) Prenylated flavonoids from Artocarpusaltilis: Antioxidant activities and inhibitory effects on melanin production. Phytochemistry 89: 78-88.
- LM Azru, R Nurulaini, MA Adzemi H Marina, AWM Effendy (2014) Tannins Quantification in Terminaliacatappa Leaves Extract and Antihelmenthic Potential Evaluation. Journal of Natural Products7: 98-103.
- Moses S Owolabi, Oladipupo A Lawal, Isiaka A Ogunwande, Rebecca M Hauser, William N Setzer, (2013) Chemical Composition of the Leaf Essential Oil of Terminaliacatappa L. Growing in Southwestern Nigeria. American Journal of Essential Oils and Natural Products 1(1): 51-54.
- Mininel FJ, Leonardo Junior CS, Espanha LG, Resende FA, Varanda EA et al. (2014) Characterization and Quantification of Compounds in the Hydroalcoholic Extract of the Leaves from Terminaliacatappa Linn. (Combretaceae) and Their Mutagenic Activity, Evidence Based Complement Alternat Med 2014: 676-902.
- Devi Ramaiya S, Bujang JS, Zakaria MH, King WS, Shaffiq Sahrir MA (2013) Sugars, ascorbic acid, total phenolic content and total antioxidant activity in passion fruit (Passiflora) cultivars. J Sci Food Agric 93(5): 1198-1205.
- Chassagne D, Crouzet JC, Bayonove CL, Baumes RL (1996) Identification and Quantification of Passion Fruit Cyanogenic Glycosides. J Agric Food Chem 44(12): 3817-3820.
- Mohammed Beltagy (2014) Investigation of new antimicrobial and antioxidant activities of Brassica rapal. International Journal of Pharmacy and Pharmaceutical Sciences 6(6): 19-25.
- Tara Kamal, Ahmad Muzammil, Muhammad Nor Omar (2012) Evaluation of antimicrobial activity of Artocarpusaltilis on pathogenic microorganisms. Science Series Data Report 4(9).
- Hesti Riasari, Maria Ulfah, Diki Prayugo Nurul Annisa Komariah (2017) Antibacterial and antifungal activities of various bread fruit leaves. International Journal of Pharmaceutical Sciences and Research IJPSR 8(3): 1066-1073.
- Jusuf NK, Putra IB, Dewi NK (2019) Antibacterial Activity of Passion Fruit Purple Variant (Passifloraedulis Sims var. edulis) Seeds Extract Against Propionibacterium acnes. Clinical, Cosmetic and Investigational Dermatology (13): 99-104.
- Chanda S, Rakholiya K, Nair R (2011) Antimicrobial Activity of Terminaliacatappa L. Leaf Extracts against Some Clinically Important Pathogenic Microbial Strains. Chinese Medicine 2: 171-177.
- Pawar SP, Pal SC (2002) Antimicrobial activity of extracts of Terminaliacatappa root, Indian J Med Sci. 56(6): 276-278.
- Nair R, Chanda S (2008) Antimicrobial Activity of Terminaliacatappa, Manilkarazapota and Piper betel Leaf Extract. Indian J Pharm Sci 70(3): 390-393.
- Ryan KJ, Ray CG (2004) Sherris Medical Microbiology (4th), McGraw Hill, USA.
- Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolke RH (1995) Manual of Clinical Microbiology. (6th), Mosby Yearbook, London.
- Chenn P, Murray J (1997) Microorganisms and Biotechnology. 1-166.
- Skoog DA, West DM, Holler FJ (1996) Fundamentals of Analytical Chemistry. (7th), Thomson Learning, Inc USA.
- Daniel HC (2003) Quantitative Chemical Analysis. (6th), W.H. Freeman and Company, New York, USA, pp. 61-79.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...