Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4706
Research ArticleOpen Access
A Comparison Study for Chances of Reduction in Cardiovascular Risk by Draksharishta in Induced Diabetic Condition Volume 1 - Issue 3
Subhajit Ghosh1*, Dr PN Murthy2 and Dr H Joshi1
- 1Sarada Vilas College of Pharmacy, India
- 2Royal College of Pharmacy and Health Sciences, India
Received: April 14, 2018; Published: April 19, 2018
Corresponding author:Subhajit Ghosh, Sarada Vilas College of Pharmacy, Mysore, Karnataka, India
DOI: 10.32474/DDIPIJ.2018.01.000111
Abstract
In this study was to evaluate the effect of Draksharishta-T and Draksharishta -M prepared by traditional and modern methods respectively and Dabur Draksharishta and comparison between them on fasting blood glucose and serum lipid profile in alloxan induced diabetic rats. Oral administration of Draksharishta-T, Draksharishta-M and Dabur Draksharishta (2ml/kg p.o.) for 21 days caused a significant decrease in fasting blood glucose (FBG) and showed significant rise in blood glutathione level (GSH) in diabetic rats. Glibenclamide was used as a standard anti diabetic drug (10 mg/kg, p.o). These preparations also caused significant reduction in serum cholesterol, LDL and triglycerides and showed significant rise in serum HDL level in diabetic albino rats. Thus all these preparations were able to maintain the tested parameters near to the normal level significantly.
Keywords: Draksharishta; Blood glucose; Anti-diabetic; Cardiovascular risk; Glutathione; Lipid profile; Alloxan
Abbrevations: FBG: Fasting Blood Glucose; GSH: Blood Glutathione Level; WHO: World Health Organization; CPCSEA: Committee for the Purpose of Control and Supervision of Experiments on Animals; ANOVA: Analysis of Variance
Introduction
Now-a-days Diabetics is a major problem of our society, not only India most of the countries because of our life style. Diabetics have accelerated levels of oxidative stress and this contributes massively to most cardiovascular, neurological, retinal and renal diabetic complications [1]. Diabetes mellitus is a heterogeneous metabolic disorder as old as mankind and its incidence is considered to be high all over the world [2]. It is characterized by hyperglycemia. Hyperglycemia significantly diminishes glutathione levels lowering defenses against oxidative stress [3]. A multitude of herbs, spices and other plant material has been described for the treatment of diabetes throughout the world [4]. Furthermore, after the recommendations made by WHO (World Health Organization) on diabetes mellitus, investigations on hypoglycemic agents from medicinal plants have become more important. The levels of serum lipids are usually elevated in diabetes mellitus and such an elevation represents the risk factor for coronary heart disease.
Moreover, diabetic patients experience a two to three fold increase in cardiovascular morbidity and mortality in comparison to non diabetics [5]. Draksharishta is a poly herbal hydro alcoholic preparation and is used as blood purifier, in the treatment of anemia and advised as a choice of remedy in respiratory problems. The chief ingredient of Draksharishta is draksha, dried fruits of Vitis vinifera [6].The composition and properties of fruits of Vitis vinifera, have been extensively investigated and it was reported that they contain large amount of phenolic compounds as catechins, epicatechin, quercetin, and gallic acid, dimeric, trimeric and tetrameric procyanidins [7]. These compounds have many favorable effects on human health such as lowering of human low density lipoproteins, reduction of heart disease and cancer [8- 11]. Therefore we undertook the present investigation to evaluate the anti diabetic effect of Draksharishta-T and Draksharishta-M prepared by traditional and modern methods respectively and also Dabur Draksharishta in alloxan induced hyperglycemic rats. Effect of these preparations was also evaluated on the serum lipid profile of alloxan induced diabetic rats.
Materials and Methods
The Health Canada (HC) website, more precisely the Drug &Health product inspections section [1] is listing 802 pages of cGMP audit results from virtually all Canadian establishment license holders. Even though compliant, the vast majority of them were listed as having inadequate quality systems. Comments such as: The handling of standard operating procedures for good manufacturing practices was inadequate, the written procedures for recalls were inadequate, the education, experience, and/ or oversight of the individual in charge of the quality control department was inadequate, to name but a few, can be read everywhere across the site. Compliant CMOs complain privately that labeling them as inadequate on the public domain, resulted in drops of direct business revenues and a weakening of their competitiveness. Indeed, foreign clients that would like to export their business in Canada are misinformed through HC website and get the impression that Canadian CMOs are problematic. In contrast FDA and EMA do not publish the same kind of data, through detailed documents and audit reports from compliant organizations. At the FDA cGMP audit reports exist and are on the public domain but recently both EMA and FDA have published the first report from the FDA-EMA pilot program for the parallel assessment of qualityby- design elements of marketing applications [2].
Preparation of Draksharishta
Draksharishta-T:This was prepared by the method as given in Ayurvedic Formulary of India [6]. The ingredients of Draksharishta were procured from Local market, Mysore. Identification of all the individual plant material was done as per Ayurvedic Pharmacopoeia of India. Authentification of all these ingredients was done in the Central National Herbarium Shibpur in West Bengal; Prepared herbarium has been deposited in the Central National Herbarium Shibpur in West Bengal for future reference. According to this method, dried fruits of Vitis vinifera after proper crushing were placed in polished vessel of brass along with prescribed quantity of water (13L), and allowed to steep overnight. After overnight steeping, this material was warmed at medium flame until the water for decoction reduced to one fourth of the prescribed quantity (3.25L), then the heating was stopped and it was filtered through unstarched muslin cloth in cleaned and fumigated vessel and after that jaggery was added and mixed properly. Then Dhataki flowers (Woodfordia floribunda) and prescribed quantity of coarsely powdered prakshepa dravyas as Cinnamomum zeyleynicum (stem bark), Eletteria cardamomum (seeds), Cinnamomum tamala (leaves), Mesua ferrea (stamens), Callicarpa macrophylla (flowers), Piper nigrum (fruits), Piper longum (fruits), Embelia ribes (fruits) were added and this sweet filtered fluid was placed for fermentation in incubator for fifteen days at 33oC±1oC.After fifteen days completion of fermentation was confirmed by standard tests [12]. The fermented preparation was filtered with unstarched muslin cloth and kept in cleaned covered vessel for further next seven days. Then, it was poured in clean amber colored glass bottles previously rinsed with ethyl alcohol, packed and labeled properly.
Draksharishta-M
Method of preparation was same as followed with Draksharishta -T, only dhataki flowers were replaced with Yeast for inducing fermentation [13].
Animals:Adult Wistar albino rats, weighing between 200- 220g of either sex were acclimatized to normal environmental conditions in the animal house for one week. The animals were housed in standard polypropylene cages and maintained under controlled room temperature (22 OC ± 2 OC) and humidity (55±5%) with 12:12 hour light and dark cycle. All the animals were given a standard chow diet (Hindustan Lever Limited), and water ad libitum. The guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) of the Government of India were followed and prior permission was granted from the Institutional Animal Ethics Committee ( 706/ CPCSEA/ dated 01.10.2002 ).
Induction of Diabetes:The animals were fasted for 18 h and made diabetic by injecting Alloxan monohydrate (150mg/ kg, i.p.) dissolved in sterile normal saline. In order to stave off the hypoglycemia during the first day, 5% w/v glucose solution was given orally to diabetic rats after four to six hours of alloxan administration. The diabetic state was confirmed when the blood sugar level was greater than 180 mg/dl [14].
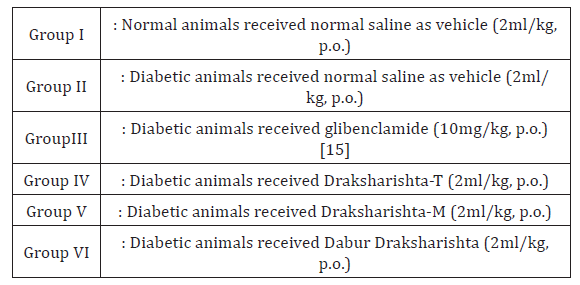
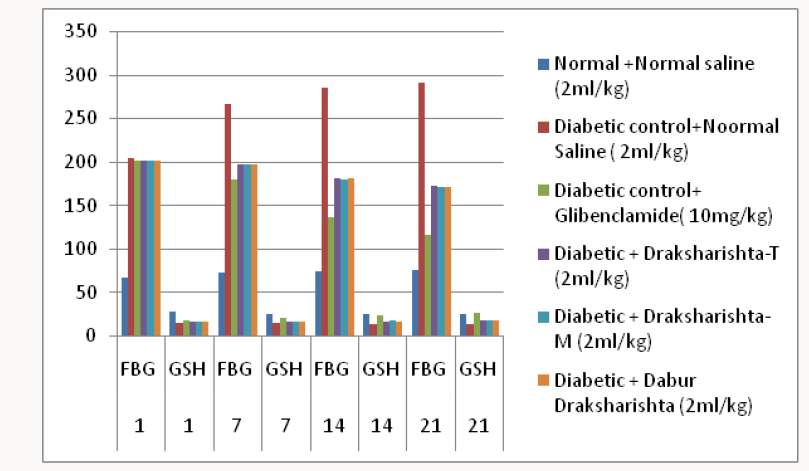
Treatment Protocol:All the animals were randomly divided into the six groups with six animals in each group. All the three types of Draksharishta as Draksharishta-T, Draksharishta-M and Dabur Draksharishta were given at a dose of 2ml/kg body weight, orally daily for a period of 21 days to different groups of diabetic animals. (Table 1) Albino rats were made diabetic by a single intra-peritoneal injection of Alloxan monohydrate (Loba Chemie, Mumbai). Alloxan was first weighed individually for each animal according to the weight and then solubilized with 0.2ml saline just prior to injection. Two days after Alloxan monohydrate injection rats with blood glucose level of greater than 180mg/dl were selected for the present study. Treatment with Draksharishta-T, Draksharishta-M and Dabur Draksharishta was started 48h after Alloxan injection. Blood samples were drawn at weekly interval until the end of study i.e three weeks. Fasting blood glucose (FBG), blood glutathione (GSH) estimation and body weight measurement were done on day 1st, 7th, 14th and 21st of the study. On 21st day blood was collected by retro orbital plexus under mild ether anesthesia and fasting blood glucose was estimated [16]. Serum was separated and analyzed for serum cholesterol [17], serum triglycerides [18], serum HDL [19], serum LDL [19], serum creatinine [20], serum urea [21], serum alkaline phosphatase [22], blood glutathione [23] by using Span and Erba diagnostic kits.
Drugs and Chemicals: Alloxan monohydrate was purchased from Loba Chemie, Mumbai. Standard anti diabetic drug glibenclamide was obtained from Ranbaxy Research Laboratories, Gurgaon, India.
Statistical Analysis: The results were expressed as mean ± SEM. Statistical analysis of data among the various groups was performed by using one way analysis of variance (ANOVA) followed by the Tukey test of significance.
Results
Effect of Different Types of Draksharishta on FBG and GSH of Diabetic Rats
All types of Draksharishta as Draksharishta-T, Draksharishta-M and Dabur Draksharishta produced significant (P<0.001) reduction in FBG level in alloxan induced diabetic rats. Glibenclamide produced significant reduction in FBG level (60%) which was highest as compared to that produced by all types of Draksharishta [Table 1]. In our study, in alloxan treated diabetic rats the blood Glutathione level (GSH) decreased significantly (P<0.001). Draksharishta-T, Draksharishta-M, Dabur Draksharishta (2ml/kg p.o.) and glibenclamide (10mg/kg p.o). Showed significant increase in GSH level on both 14th and 21st day of treatment (Table 2)(Figure 1).
Table 2: Effect of Draksharishta-T and Draksharishta-M on Fasting blood glucose and Blood glutathione level of diabetic rats.
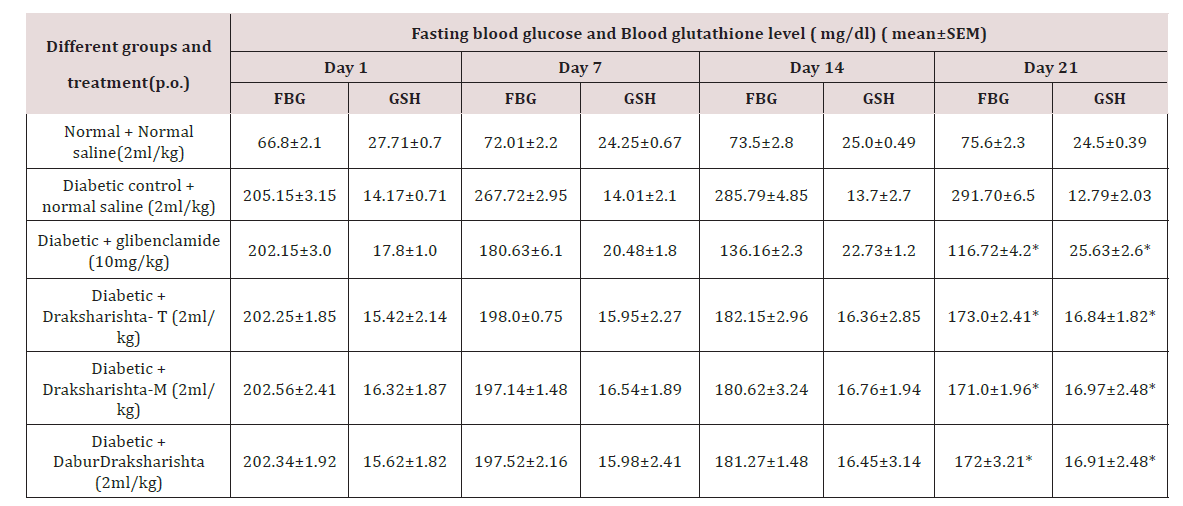
*P< 0.001 as compared to diabetic control group, n = 6 in each group.
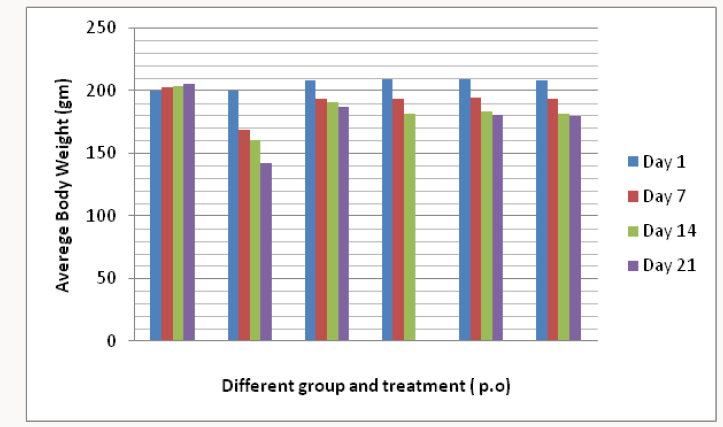
Effect of Different Types of Draksharishta on Body weight of Diabetic Rats
All the Draksharishta preparations as Draksharishta-T, Draksharishta -M, Dabur Draksharishta and glibenclamide exhibited significant antihyperglycemic activity in alloxan induced diabetic rats without causing significant change in body weight (Table 3) (Figure 2).
Table 3: Effect of Draksharishta-T and Draksharishta- M on body weight of alloxan induced diabetic rats.
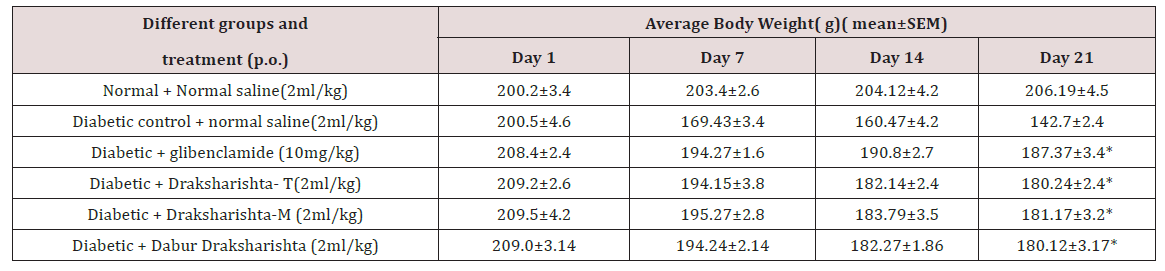
*P< 0.001 as compared to diabetic control group, n = 6 in each group.
Effect of Different Types of Draksharishta on Serum Lipid Profile of Diabetic Rats
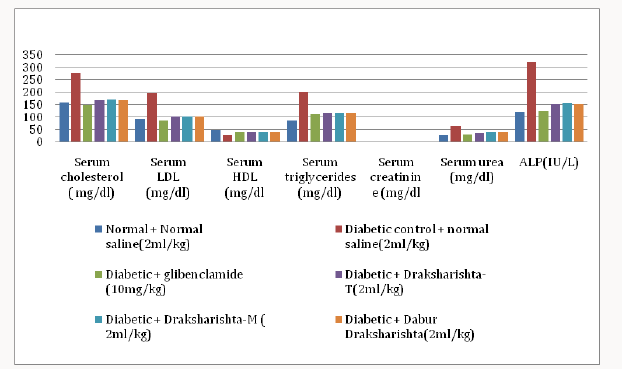
Draksharishta-T and Draksharishta-M at the dose of 2ml/kg body weight orally significantly reduced total serum cholesterol (40% and 39%), serum LDL (48% and 48%) and triglycerides (42% and 41%) when compared to diabetic control. The increase in HDL was (36% and 34%) with Draksharishta-T and Draksharishta-M respectively. Draksharishta -T and Draksharishta-M also significantly reduced serum creatinine (64% and 63%), serum urea (44% and 42%) and serum alkaline phosphates levels (53% and 52%). Dabur Draksharishta produced similar effects on the serum lipid profile of diabetic rats as that of Draksharishta- T and Draksharishta-M whereas glibenclamide caused highest significant (P<0.001) reduction in the levels of serum cholesterol, triglyceride and LDL as well as significant improvement in the level of HDLcholesterol in diabetic rats (Table 4) (Figure 3).
Table 4: Effect of Draksharishta-T and Draksharishta-M on serum lipid profile of diabetic rats after three weeks of treatment.
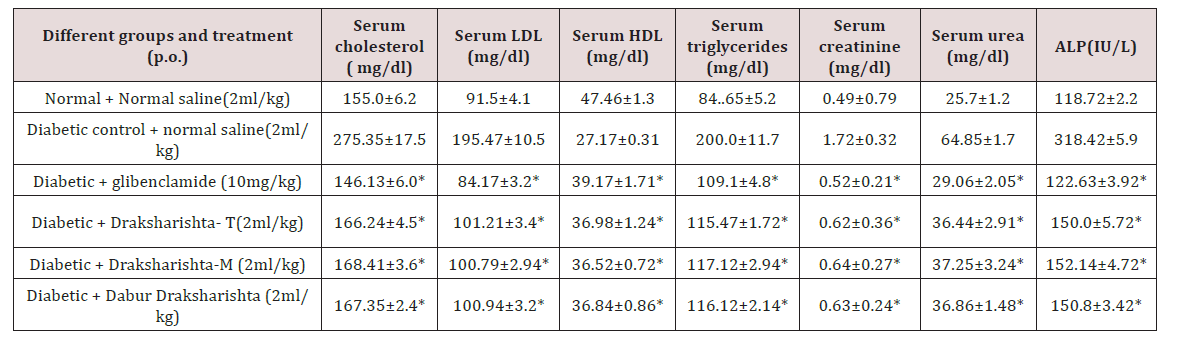
All values are expressed as mean ± SEM, n = 6 in each group; *P < 0.001 as compared to diabetic control.
Discussion
Administration of alloxan caused rapid destruction of pancreatic ß cells in rats, which led to impaired glucose stimulated insulin release and insulin resistance, both of which are marked feature of type II diabetes [24]. This is because administration of alloxan (150mg/kg i.p.) led to more than 1.5 fold elevation of fasting blood glucose level, which was maintained over a period of three weeks. Three week of daily treatment of Draksharishta-T, Draksharishta-M and Dabur Drakshrishta (2ml/kg p.o.) caused significant fall in blood glucose level. GSH, being the most important bio-molecule against chemically induced toxicity can participate in the elimination of reactive intermediates by reduction of hydroper- oxidase in the presence of glutathione per-oxidase. The most important mechanism implicated in the diabetogenic action of alloxan is by increased generation of oxygen free radicals which cause a decrease in plasma GSH concentration. Hence, drugs that could prevent the generation of these oxygen free radicals or increase the free radical scavenging enzymes may be effective in alloxan induced diabetes [25]. In the present study, the observed significant increase in blood glucose level and a decrease in blood glutathione levels in diabetic rats could be due to destruction of ß-cells by alloxan reinforcing the view that alloxan induced diabetes probably through the generation of oxygen free radicals [26].The standard anti-diabetic drug glibenclamide and the test preparations as Draksharishta-T, Draksharishta-M and Dabur Draksharishta showed that they could prevent the development of Diabetes mellitus in albino rats, due to their antioxidant property since they showed a significant decrease in fasting blood glucose level (FBG) along with significant increase in blood glutathione level (GSH) after treatment Vehicle control animals were found to be stable in their body weight but diabetic rats showed significant reduction in body weight during 21 days [Table 2]. Alloxan caused body weight reduction, which was improved by standard drug (glibenclamide) and test preparations (Draksharishta-T, Draksharishta-M and Dabur Draksharishta) nearly equal to the normal. In general, an increase in blood glucose level is usually accompanied by an increase in plasma cholesterol, triglyceride, LDL levels and a decrease in HDL levels as observed in diabetic patients [27]. The marked hyperlipidemia that characterizes the diabetic state may be the consequence of the uninhibited actions of lipolytic hormones on fat depots [28]. Draksharishta-T, Draksharishta-M, Dabur Draksharishta and standard anti diabetic glibenclamide produced significant reduction in fasting blood glucose level. These Draksharishta preparations and glibenclamide also produced significant reduction in serum cholesterol, LDL and triglyceride along with significant rise in HDL level in alloxan induced diabetic rats. The improvement in the lipid profile in diabetic animals after treatment with Draksharishta-T, Draksharishta-M, Dabur Draksharishta and standard antidiabetic glibenclamide could be beneficial in preventing diabetic complications, as well as improving lipid metabolism in diabetic patients [29]. A decrease in the FBG level, improvement in the lipid profile along with significant rise in the blood glutathione level (GSH) by Draksharishta-T, Draksharishta-M and Dabur Draksharishta in alloxan induced diabetic rats suggests that these preparations could be useful as an anti-diabetic agent with cardioprotective activity. The obtained result suggests that presence of alcohol could be beneficial in the faster absorption of poly phenolic compounds found present in Draksharishta which are responsible for showing scavenging of alloxan induced reactive oxygen species.
Acknowledgement
The authors are immensely thankful to the Department of Pharmaceutical Chemistry, Pharmacology for providing the requisite facilities.
References
- Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H , et al. (2000) Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Met 85(8): 2970-2973.
- Pickup JC, William G (1997) Epidemiology of Diabetes mellitus. In Textbook of Diabetes. Vol I, 2nd edn. Blackwell, Oxford p. 3.1-3.28.
- Sagara M, Satoh J, Wada R, Yagihashi S, Takahashi K, et al. (1996) Inhibition of development of peripheral neuropathy in STZ- induced diabetic rats with N-acetylcysteine. Diabetologia 39(3): 263-269.
- Kesari AN, Gupta RK, Watal G (2005) Hypoglycemic effects of Murraya koenigii on normal and alloxan diabetic rabbits. J Ethnopharmacol 97(2): 247-251.
- Davidson MB (1981) Diabetes mellitus Diagnosis and treatment. New York, UK. Wiley p. 27-48.
- (2000) The Ayurvedic Formulary of India Part-II. Delhi, India: Controller of Publications p. 15-16.
- Baydar NG, Ozkan G, Sagdic O (2004) Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control 15(5): 335-339.
- Frankel EN, Kanner J, German JB, Parks E, Kinsella JE (1993) Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. The Lancet 341(8843): 454-457.
- Mayer AS, Yi OS, Person DA, Waterhouse DL, Frankel EN (1997) Inhibition of human low-density lipoprotein oxidation in relation to composition of phenolic antioxidants in grapes (Vitis vinifera). J Agri Food Chem 45(5):1638-1643.
- Teissedre PL, Frankel EN, Waterhouse AL, Peleg H, German GB (1996) Inhibition of in vitro human LDL oxidation by phenolic antioxidants from grapes and wines. J Sci Food Agri 70: 55-61.
- Waterhouse AL (1994) Wine antioxidants may reduce heart disease and cancer. Presentation of American Chemical Society, Washington.
- Mishra S (2005) Bhaisazya Kalpana Vigyan. Varanasi, India: Chaukambha Bharati Prakashan pp. 253-254.
- Alam M, Radhamani S, Ali U, Purushottam KK (1984) Microbiological Screening of Dhataki Flowers. J Res Ayurveda Siddha 2(4): 371-375.
- Nishikant AR, Naresh JG (2006) Antidiabetic Activity of hydro-ethanolic extract of Cyperus rotundus in alloxan induced Diabetes in rats. Fitoterapia 77(7-8): 585-588.
- Sharma SR, Dwivedi SK, Swarup D (1996) Hypoglycemic and hypolipidemic effects of Cinnamomum tamala Nees leaves. Indian J Exp Biol 34: 372-374.
- Giordano BP, Thrash W, Hollenbaugh L, Dube WP, Hodges C, et al. (1989) Performance of seven blood glucose testing systems at high altitude. Diabet Educa 15(5): 444-448.
- Allain CC, Poon LS, Chan CS, Richmond W (1974) Enzymatic Determination of Total Serum cholesterol. Clin Chem 20(4): 470-475.
- Muller PH, Schmulling RM, Liebich HM, Eggstein M, Stahler F, et al. (1977) A fully Enzymatic Triglyceride Determination in a continuous flow 12-channel analyzer. J Clin Chem 15(9): 503-508.
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the Concentration of Low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6): 499-502.
- Bowers LD (1980) Kinetic Serum Creatinine Assay. The role of various factors in determining specificity. Clin Chem 26(5): 551-554.
- Wilson BW (1966) Automatic Estimation of urea using urease and alkaline phenol. Clin Chem 12(6): 360-368.
- Sasaki MA (1966) New method for the determination of serum alkaline phosphatase. Use of Berthelot’s reaction for the estimation of phenol released by enzymatic activity. Igaku to Seibutsugaku 70: 208-214.
- Beutler E, Duron O, Kelly BM (1963) Improved method for determination of blood glutathione. J Lab Clin Med 61: 882-888.
- Proks P, Reimann F, Gribble F (2002) Sulfonyl urea stimulation in insulin secretion. Diabetes 51(Suppl 1): S368-S376.
- Shankar PK, Kumar V, Rao N (2005) Evaluation of Anti-diabetic activity of Ginkgo biloba in streptozotocin induced Diabetic Rats. Iranian J Pharmacol Therapeutics 4(1): 16-19.
- Wohaleb SA, Godin DV (1987) Alterations in free radical tissue defense mechanism in streptozotocin induced diabetes in rat. Effects of insulin treatment. Diabetes 36(9): 1014-1018.
- Mitra SK, Gopumadhvans, Muralidhar TS, Anturlikar SD, Sujatha MB (1995) Effect of D-400, a herbomineral preparation on lipid profile, glycosylated haemoglobin and glucose tolerance in streptozotocin induced diabetic rats. Indian J Exp Biol 33: 798-800.
- Bopanna KN, Kanna J, Sushma G, Balaram R, Rathod SP (1997) Antidiabetic and antihyperlipidemic effects of neem seed kernel powder on alloxan diabetic rabbits. Indian J Pharmacol 29(3): 162-167.
- Cho SY, Park JY, Park EM, Choi MS, Lee MK, et al. (2002) Alteration of hepatic antioxidant enzyme activities and lipid profile in streptozotocin induced diabetic rats by supplementation of dandelion water extract. Clin Chem Acta 317(1-2): 109-117.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...