Lupine Publishers Group
Lupine Publishers
Menu
Research Article(ISSN: 2641-6875) 
The Efficacies of a Novel Formulation called WuFeiZi® on the Improvement of Hair Conditions Regarding Hair Loss and Hair Density Volume 2 - Issue 2
Rumeng Jin1,2, Weicheng Fei3, Dan Cheng4*
- 1Mama Mujer (Beijing) Technology Co., Ltd, Beijing, China
- 2Mama Mujer R&D Team, Beijing, China
- 3Huiwen Biotechnology Co., Ltd, Shanghai, China
- 4North Carolina A&T State University, Greensboro, US
Received: May 15, 2023; Published: May 24, 2023
*Corresponding author: Dan Cheng, North Carolina A&T State University, Greensboro, US
DOI: 10.32474/CTBM.2023.03.000169
Abstract
Hair loss, commonly observed as a thinning or shedding of hair caused by the early entry of the hair from anagen phase into the telogen phase, has caused a lot of anxiety. While treatments for hair loss consist of pharmaceutical products, physical and regenerative medical therapies, they might lead to side-effects and be expensive. In our clinical study, we aimed to demonstrate the efficacies of a new formulation composed of natural and functional food ingredients on preventing hair loss. After orally taking this functional food for 4 weeks, the average hair loss of the subjects significantly reduced by 32.13%. The overall hair density increased by 2.2% while the local hair density significantly increased by 6.61%. The trans-epidermal water loss for the scalp reduced by 1.88%. The satisfaction assessment from the subjects were high regarding efficacy, safety, taste and willingness for continued supplementation. In particular, the 4 week follow-up observation of the individuals concluded that there were no side effects if the administration of this product was suspended. This product should have been valuable and filled the huge vacancy of individuals bothered by hair loss.
Introduction
In China, hair loss has been a common problem leading to aesthetic discomfort and anxiety especially for young individuals. While androgenic alopecia is the most common type of hair loss, more individuals are mainly concerned by a thinning or shedding of hair caused by the early entry of the hair from anagen phase into the telogen phase. The reasons include unhealthy life styles, pregnancy and menopause with hormonal changes that result a hair loss with increased prevalence with aging [1,2]. Hair growth is a complex process in which the hair follicle undergoes three phases of growth (anagen), apoptosis-driven regression (catagen) and resting (telogen). The anagen phase, is the longest period of the hair life cycle, lasting one to six years on average, when a massive keratinocyte proliferation in the hair matrix occurred, followed by a rapid cellular differentiation with pigmentation by follicular melanogenesis. The catagen phase is a regression phase, lasting for a few weeks, during which the hair stops growing. The telogen phase of 3-9 months refers to the “resting” phase during which the hair no longer grows. At the end of this phase, keratinized hair falls out and a new hair starts to grow, and the follicle goes back in the anagen phase [3]. For a normal hair life cycle, the percentages of hair in the anagen phase, catagen phase and telogen phase are between 85% and 90%, approximately 1%, and approximately 10 to 20% of the total hair respectively [4]. Growth and differentiation of the matrix cells are under the influence of growth factors produced by the dermal papilla [5,6], including the epidermal growth factor (EGF)- related ligands, fibroblast growth factors (FGF), transforming growth factor-beta (TGF-beta), insulin-like growth factor (IGF), due to their crucial regulation of the hair cycle and hair growth [7]. The highly vascularized dermal papilla, considered as the “biological engine” of the hair, is a hair bulb under the scalp surface, composed of fibroblasts that provide fundamental hair nutrition, ensure irrigation, oxygenation, and dispose cellular waste.
Available treatments for hair loss consist of pharmaceutical products, physical and regenerative medical therapies, and hair transplant procedures. For instance, Finasteride, a class of medications called 5-alpha reductase inhibitors, is used to treat male pattern hair loss (gradual thinning of the hair on the scalp, leading to a receding hairline or balding on the top of the head in men). However, a review showed that finasteride is infrequently associated with problems of ejaculation (2.1-7.7%), erection (4.9- 15.8%), and libido (3.1-5.4%) [8]. Nutraceuticals by providing essential nutrients or specific botanical extracts can be effective strategies to prevent or to treat weak to moderate hair loss [9]. For individuals especially sophisticated consumers in China, natural, healthy, safe and tasty functional food instead of medicines and supplements as tablets or capsules are much more welcomed, for which the trending has been dramatically increased in recent years. In our clinical study, we aimed to demonstrate the efficacies of a new formulation composed of functional food ingredients on hair loss reduction based on the previous in vitro studies this new formulation can boost certain gene expressions such as FGF-7 for hair growth. The primary outcomes of the clinical trial were the overall and local density of the hair. The secondary endpoints were the trans-epidermal loss of the skin and the general self-assessment by the volunteers.
Materials and Methods
Intervention
20 subjects who are from 18-60 years old with hair length between 5~40 cm but had issues of hair loss and slight thinning of hair were enrolled for the formal trial to ensure the balance of important factors (gender, age, hair length, hair loss severity, etc.) that may affect the test results. The product is a liquid drink called WuFeiZi® mainly consisting of pea seedling extract, collagen peptides, sea cucumber peptides, B vitamins and so on. The testing cycle of 10 weeks include 2 weeks wash-out period, 4 weeks intervention, followed by 4 weeks observational study for safety and satisfaction concerns. Before the enrollment, subjects were asked a series of questions about disease history, health status and so on, and were required to sign a written informed consent form. During the trial period, subjects should not wash their hair within 48 ± 4 hours before each visit, which should be basically kept consistent for each visit. They should not comb their hair on the day of the visit. Also, no haircut within 2 weeks was allowed before each visit. During the trial period, no hair care and hairdressing measures can be taken, nor can any treatment be received for hair growth. It was necessary to maintain original living habits and avoid large emotional fluctuations.
Measurement
The hair evaluation of the subjects were conducted before and after using the product, regarding hair density evaluation and image shooting. Hair density assessment includes overall hair density assessment and local hair density assessment. Also, for each visit, a trained staff combed the hair of the subjects 60 times, and then counted and recorded the hair loss.
Overall, Hair Density
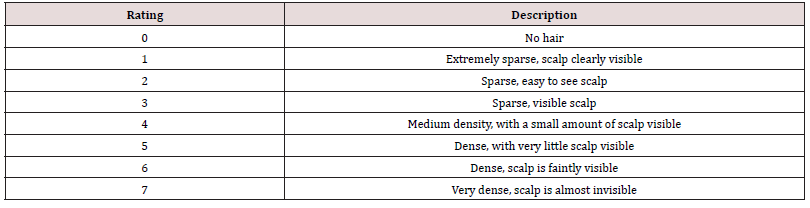
The hair on the head of the subjects was combed symmetrically to both sides and kept smooth. The overall hair density evaluation includes visual evaluation and image evaluation by a dermatologist using the 0-7 evaluation scale in Table 1. For image evaluation, each subject wore a black non-reflective scarf and combed the hair smoothly, and then the chin was placed on the shooting bracket. Photos of the whole head with the head as the center were taken. A standard color palette (at least black, white and gray) was also set. After the visit, a dermatologist would evaluate the hair density of the photos taken and record the grading. The average value of visual evaluation and image evaluation was calculated as the overall hair density.
Local Hair Density
For local hair density, a hair area of at least 1.5 cm × 1.5 cm on the head of the subjects was well positioned and cut, leaving the residual length for no more than 1 mm. In the process of image acquisition, the operator kept the subject in a comfortable position, place the dermal scope vertically above the center of the removal area for local hair image shooting. Image analysis software or manual counting method were used to count and record the number and density of local hair.
Scalp Dehydration
Before and after 4 weeks trial, the scalp dehydration was tested by Tewameter TM300 for the transcutaneous water loss in the scalp hair cutting area of the subjects.
Subjective Assessment
After the trial was complete, the subjective evaluations for the product regarding its taste, efficacy and repurchase willingness were conducted. A follow-up questionnaire was issued to investigate the hair loss, adverse event and other relevant feelings during the 4 weeks after the intervention was complete.
Statistical Analysis
Statistical analysis software was used for statistical analysis of data. A normal distribution test is conducted and paired t test is used for the comparison before and after the measurement. Otherwise, the rank sum test of two related samples was used. The above statistical analysis was based on a two-tailed test, with a significance level of α= 0.05.
Results
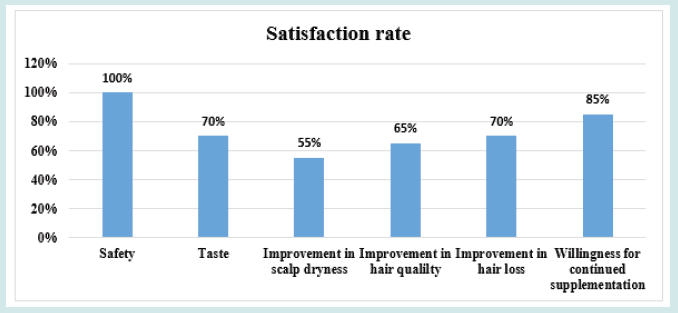
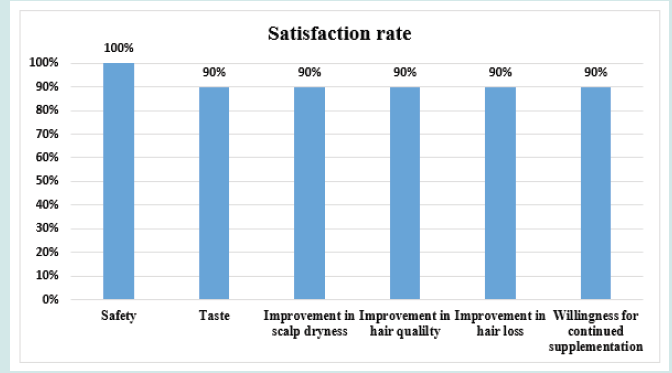
After orally taking WuFeiZi® liquid drink for 4 weeks, the average hair loss of the subjects significantly reduced by 32.13% (Figure 1, p<0.001). The overall hair density increased by 2.2% but with no statistical significance (Figure 2, p>0.05). However, it is obvious some individuals had improvement in overall hair density (Figure 3). The local hair density was significantly increased by 6.61% (Figure 4, p<0.01- Figure 5). The trans-epidermal water loss for the scalp reduced by 1.88% (Figure 6, p>0.05) The feedback records of the self-assessment questionnaire for 20 subjects after taking the product are positive and impressing (Figure 7). 100% of the subjects believed that the product was safe and healthy and were satisfied. 70% of the subjects believed that the product had a good taste. 55.00% of the subjects believed that the product had the effect of improving scalp dryness. 65.00% of the subjects believed that the product had the effect of improving hair quality. 70.00% of the subjects believed that the number of hair loss decreased after using the product. 85.00% of the subjects were willing to continue using the product. The 28 days follow-up for assessing the satisfaction of taking such a product showed to be more promising due to much higher satisfaction for the product (Figure 8).
Figure 1: The improvement of hair loss n.s. denotes not significant, *denotes significant differences (0.01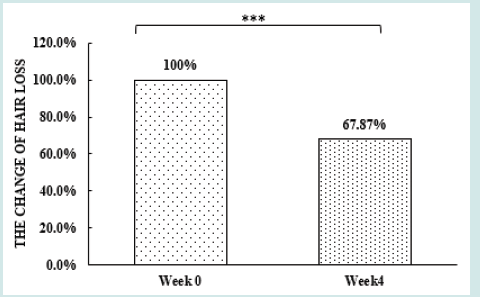
Figure 2: the improvement of overall hair density n.s. denotes not significant, *denotes significant differences (0.01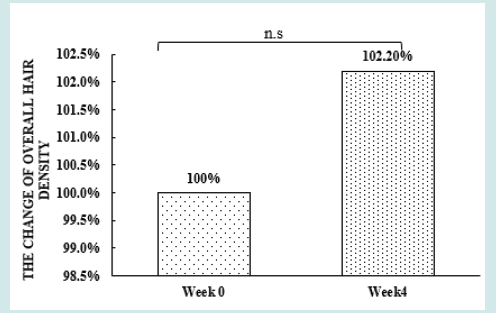
Figure 4: the improvement of local hair density n.s. denotes not significant, *denotes significant differences (0.01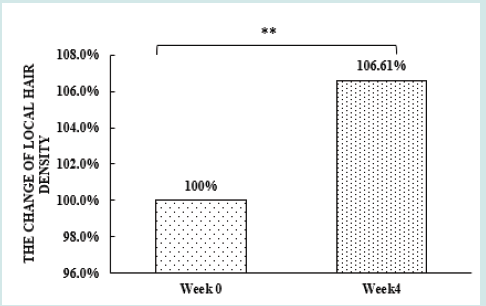
Figure 5: the local hair density for a subject: it increased from 165.6/cm2 to 177.4/cm2 after 4 weeks intervention.
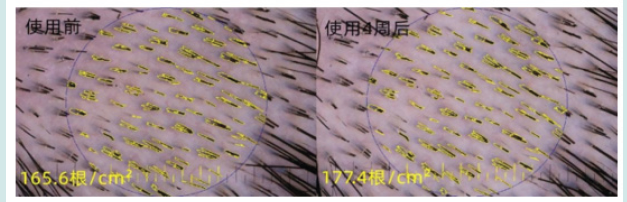
Figure 6: the improvement of trans-epidermal water loss n.s. denotes not significant, *denotes significant differences
(0.01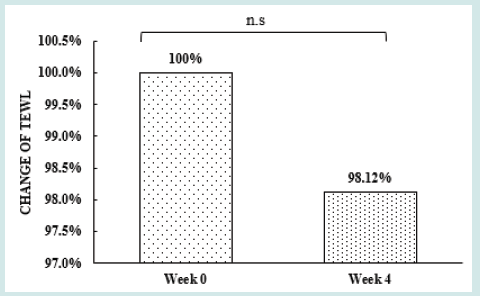
Discussion
Telogen hair loss is a physiological form of hair loss but can become chronic if periods of hair shedding last for more than 6 months. Disturbances of the hair cycle lead to increased proportion of hair shifts from the anagen phase to the telogen phase, which takes up to 30% or more of the total hair. However, most of the treatments target pathological and hormonal hair loss using medicines while treatments for the physiological and non-hormonal telogen hair loss are scarce, not to mention the side effects from medicines. In our clinical study, we proposed an innovative, alternative, and nutritional solution for reversible hair loss caused by the abnormal hair cycle. For individuals with telogen hair loss, we demonstrated that a 4-week supplementation of WuFeiZi® liquid drink significantly reduced the hair loss almost by 33% while the local hair density was significantly increased by 6%. The product contained a natural herbal extract from pea seedling which contained a lot of nutrients such as B vitamins and adenosines for survive this vulnerable phase [10]. In a clinical study with 20 volunteers, it was clearly found the number of hairs in the anagen phase was increased and he amount of telogen hair was reduced by orally administration of this pea seedling extract, which significantly increase several times the expression of fibroblast growth factor-7 (FGF7), a dermal papilla signal instructing hair germ cells to proliferate and initiate a new hair cycle [11]. On the other hand, noggin, a gene that shortens the telogen phase and initiates a new hair growth phase was even more stimulated. Moreover, the collagen peptides and sea cucumber peptides contained in this product might stimulate the proliferation of the fibroblasts that synthesize extracellular matrix for hair growth. On the other hand, after the trial was complete, the satisfaction assessment from the subjects were high regarding efficacy, safety, taste and willingness for continued supplementation. In particular, the 4 week follow-up assessment for the safety of this product brought the conclusion that there were no side effects after the administration of this product was suspended. Due to the tremendous needs of healthy hair for individuals, while there is a lack of effective, safe and tasty functional food, this product should have been valuable and filled the huge vacancy.
References
- Messenger AG (2011) Hair through the female life cycle. The British journal of dermatology 165 Suppl 3: 2-6.
- Shrivastava SB (2009) Diffuse hair loss in an adult female: approach to diagnosis and management. Indian journal of dermatology, venereology and leprology 75(1): 20-28.
- Harland DP (2018) Introduction to Hair Development. Advances in experimental medicine and biology, 1054: 89-96.
- Paus R (1998) Principles of hair cycle control. The Journal of dermatology 25(12): 793-802.
- Wilson CL, Sun TT, Lavker RM (1994) Cells in the bulge of the mouse telogen follicle give rise to the lower anagen follicle. Skin pharmacology: the official journal of the Skin Pharmacology Society, 7(1-2): 8-11.
- Rochat A, Kobayashi K, Barrandon Y (1994) Location of stem cells of human hair follicles by clonal analysis. Cell 76(6): 1063-1073.
- Peus D, Pittelkow MR (1996) Growth factors in hair organ development and the hair growth cycle. Dermatologic clinics 14(4): 559-572.
- Mysore V (2012) Finasteride and sexual side effects. Indian dermatology online journal 3(1): 62-65.
- Keophiphath M, Courbière C, Manzato L, Lamour I, Gaillard E,er al. (2020) "Miliacin encapsulated by polar lipids stimulates cell proliferation in hair bulb and improves telogen effluvium in women". Journal of cosmetic dermatology 19(2): 485-493.
- Oura H, Iino M, Nakazawa Y, Tajima M, Ideta R, et al. (2008) Adenosine increases anagen hair growth and thick hairs in Japanese women with female pattern hair loss: a pilot, double-blind, randomized, placebo-controlled trial. The Journal of dermatology 35(12): 763-767.
- Grothe T, Wandrey F, Schuerch C (2020) Short communication: Clinical evaluation of pea sprout extract in the treatment of hair loss. Phytotherapy research : PTR 34(2): 428-431.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...